Organoids are three-dimensional multicellular tissue structures that mimic the structure and function of corresponding endogenous organs. Organ-specific cells derived from human stem cells can self-organize to form organoids. Two types of stem cells are primarily used for organoid preparation. These include pluripotent embryonic stem cells or induced pluripotent stem cells and organ-restricted stem cells.
Organoids have shown promising outcomes in understanding the pathogenesis of respiratory and intestinal infections. For example, during the Zika virus outbreak, brain organoids developed from induced pluripotent stem cells have been used widely to understand disease pathogenesis.
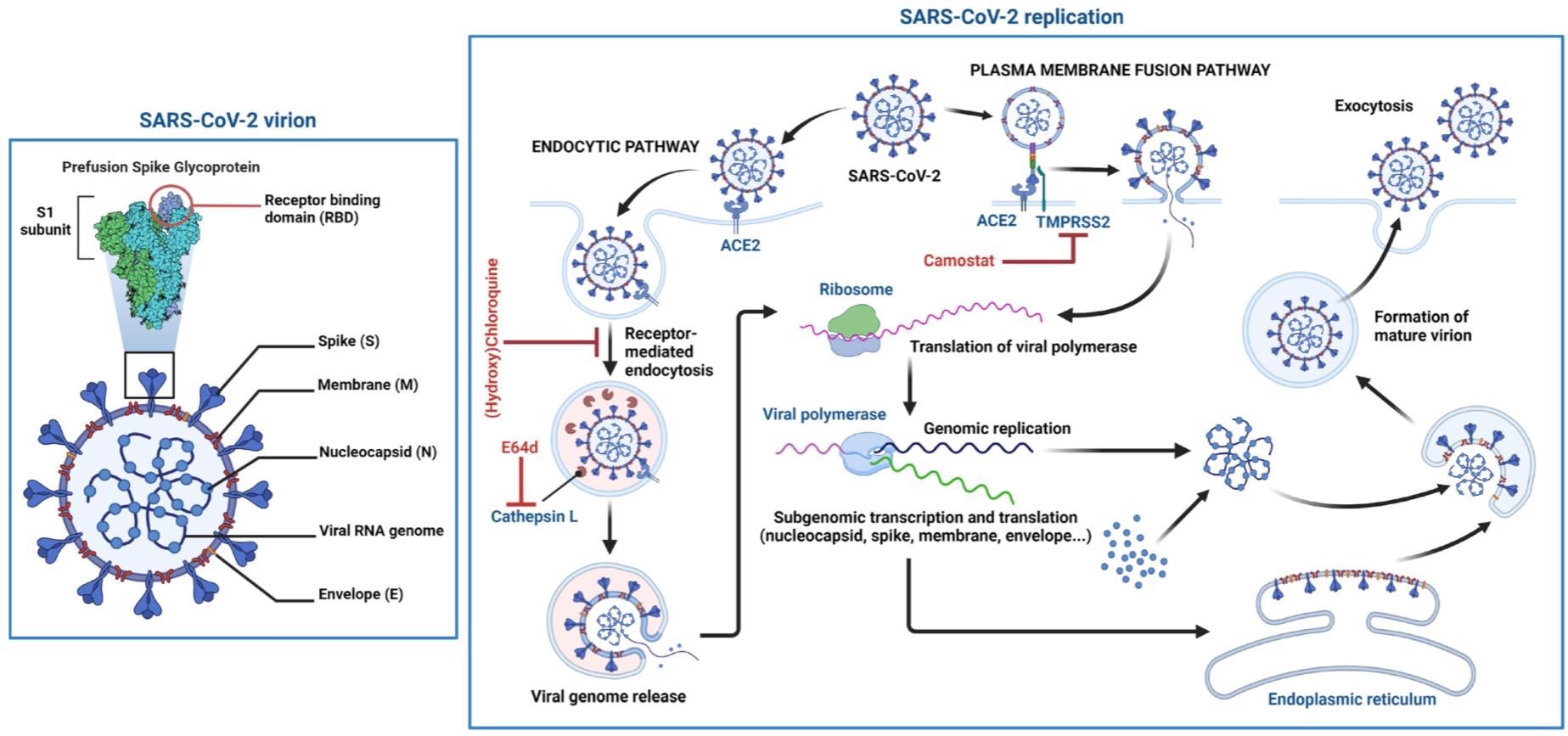 Schematic structure of the SARS-CoV-2 virion and the viral life cycle. The SARS-CoV-2 virion contains an envelope with three proteins: the spike (S) protein, membrane (M) and envelope (E) proteins. The viral RNA genome and the nucleocapsid (N) protein are contained inside the particle. The virion schematic shows in more detail the S protein subunit 1 (S1) containing the receptor-binding domain (RBD). The SARS-CoV-2 replication cycle shows two modes of viral entry (endocytic and TMPRSS2-mediated pathways). Both require docking to ACE2 at the cell surface and release of the viral genome into the cytoplasm via membrane fusion. The endocytic pathway is inhibited by (hydroxy)chloroquine and E64d, while TMPRSS2-mediated entry is inhibited by camostat. After synthesis of viral genome and proteins, viral particles are assembled and exit the infected cell.
Schematic structure of the SARS-CoV-2 virion and the viral life cycle. The SARS-CoV-2 virion contains an envelope with three proteins: the spike (S) protein, membrane (M) and envelope (E) proteins. The viral RNA genome and the nucleocapsid (N) protein are contained inside the particle. The virion schematic shows in more detail the S protein subunit 1 (S1) containing the receptor-binding domain (RBD). The SARS-CoV-2 replication cycle shows two modes of viral entry (endocytic and TMPRSS2-mediated pathways). Both require docking to ACE2 at the cell surface and release of the viral genome into the cytoplasm via membrane fusion. The endocytic pathway is inhibited by (hydroxy)chloroquine and E64d, while TMPRSS2-mediated entry is inhibited by camostat. After synthesis of viral genome and proteins, viral particles are assembled and exit the infected cell.
Organoids in COVID-19
SARS-CoV-2 is an enveloped, single-stranded, positive-sense RNA virus belonging to the human beta-coronavirus family. Being a respiratory virus, it primarily infects airway epithelial cells, causing infections in the upper and lower respiratory tracts. However, the virus also induces infections in blood vessels, heart, intestine, brain, liver, and kidney in severe cases.
The organoid technology has played an immensely vital role in understanding the life cycle and cellular tropism of SARS-CoV-2, host-virus interactions, and pathogenesis of multisystem COVID-19. Various organ-specific organoids have been used in COVID-19 related studies to understand the disease pathology in specific tissues.
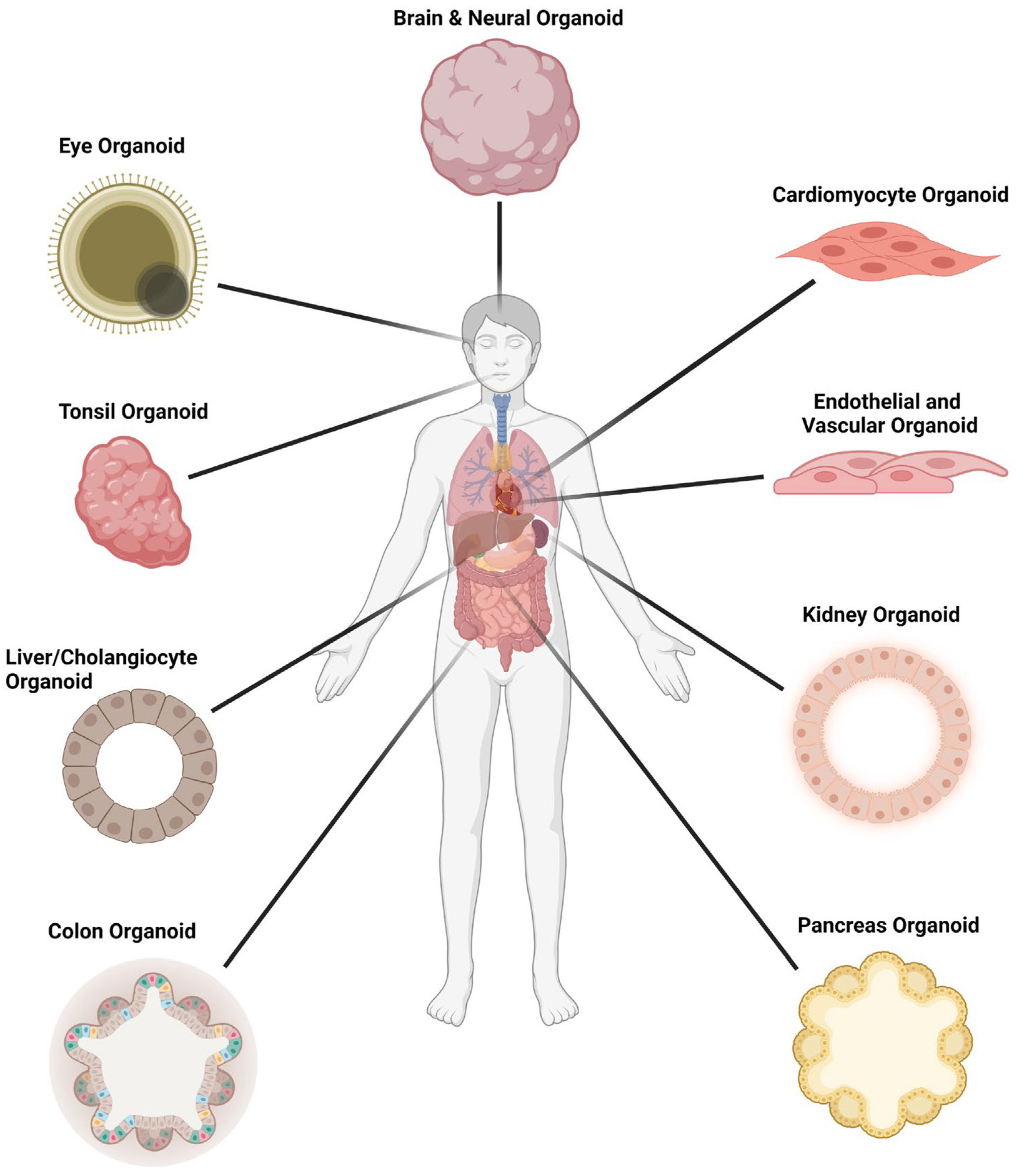 Organoid models used in SARS-CoV-2 infection studies.
Organoid models used in SARS-CoV-2 infection studies.
Organoid models of the respiratory tract
Respiratory organoids developed from progenitor cell-derived human airway epithelium have been used to understand the cellular tropism of SARS-CoV-2 in the respiratory tract. These organoids can be differentiated at an air-liquid interface or embedded into a matrix. Several types of cells are included in respiratory organoids, including basal cells, ciliated cells, and goblet cells.
Studies utilizing respiratory organoids have confirmed that SARS-CoV-2 predominantly infects ciliated cells and the viral entry and release occur from the apical airway surface. In addition, it has been observed that the expressions of angiotensin-converting enzyme 2 (ACE2) and TMPRSS2 (host cell proteins facilitating viral entry) are significantly high in the nose epithelium, which reduce gradually towards the proximal respiratory tract. Studies using lung bud organoids have demonstrated that type II pneumocytes support SARS-CoV-2 infection in the alveolar region.
Several organoid cultures established from respiratory tissues are now commercially available, including nose, bronchial, alveolar, and lung organoids. Despite being expensive, commercial organoids play immensely important roles in drug and vaccine development against COVID-19.
Organoid models of intestinal epithelium
Adult intestinal epithelial stem cells have been used to develop human gut organoids to study the pathogenesis of SARS-CoV-2 infection in the gastrointestinal tract. Using human small intestinal organoids together with alveolar organoids, the difference in replication capacity between the original SARS-CoV-2 strain and the alpha variant has been established.
Because of the high expression of ACE2 and TMPRSS2, small intestinal organoids have been used extensively to understand the wide range of transmission and infection dynamics of SARS-CoV-2 as well as virus-induced changes in the gut microbiota. In addition, these organoids have been used to screen potential antiviral agents.
Organoid models of the cardiovascular system
Induced pluripotent stem cell-derived cardiomyocytes have been used to develop heart organoids. Studies using these organoids have shown that SARS-CoV-2 entry into the cardiomyocytes requires interaction with ACE2; however, the entry can proceed via the endocytic or TMPRSS2-dependent pathway. These organoids have also been used to identify SARS-C-V-2 inhibitors and understand the cardiac damages caused by the virus.
Induced pluripotent stem cell-derived capillary organoids have been used to confirm productive SARS-CoV-2 infection in endothelial cells. In addition, in COVID-19 patients, a significant association has been observed between circulating endothelial cells and disease severity.
To understand the wide range of cardiovascular pathologies related to COVID-19, more complex organoids, wherein the vasculature is embedded within the organ, are required.
Organoid models of brain
Human embryonic stem cell- or induced pluripotent stem cell-derived brain organoids have been used to understand the mechanism of SARS-CoV-2 entry into the central nervous system as well as virus-induced neurological complications, including headache, dizziness, loss of smell and taste, seizures, encephalopathy, and cerebrovascular disease. Various region-specific brain organoids, including cortical, hippocampal, hypothalamic, and mid-brain organoids, have been developed. However, these organoids failed to confirm productive SARS-CoV-2 infection in the brain.
A production brain infection has been confirmed by using brain organoids containing choroid plexus epithelium. This model has shown that SARS-CoV-2-induced epithelial damage leads to loss of barrier function, facilitating viral entry into the brain.
Organoid models of kidney
Engineered kidney organoids expressing ACE2 and TMPRSS2 have been developed to understand SARS-CoV-2 induced kidney pathologies. Similarly, normal human kidney proximal tubule epithelial cells have been used to develop kidney epithelium organoids embedded in a matrix. To develop kidney organoids, conditional reprogramming conditions have been used to initially create two-dimensional long-term cultures, followed by their differentiation into three-dimensional organoids.
Organoid models of eye
The ocular surfaces, including the cornea, sclera, and limbus, are considered alternative sites of SARS-CoV-2 infection. Human embryonic stem cell- induced pluripotent stem cell-derived retinal and “whole eye” organoids- has been used to study ocular SARS-CoV-2 infection. These studies have confirmed the expression of ACE2 and TMPRSS2 and SARS-CoV-2 infection in limbus cells.
Collectively, a wide range of human organoid pre-clinical models developed during the COVID-19 pandemic provides a valuable platform to study viral life cycle, transmissibility, and pathogenesis.