The global coronavirus 2019 (COVID-19) pandemic has affected nearly every country in the world, as over 6.1 million deaths have been reported due to COVID-19. The development and mass administration of vaccines have allowed many developed nations to begin recovering from the current pandemic; however, developing nations have yet to receive sufficient vaccine doses to boost the immunity of their own citizens.
Newly emerging variants of the causative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also continue to show the ability to evade both vaccine-induced and natural immunity, thus highlighting the need for further research into vaccines and immunity. In a recent study published on the preprint server bioRxiv*, researchers report the results for a new SARS-CoV-2 vaccine currently in Phase III clinical trial known as I53-50.
Study: Durable protection against SARS-CoV-2 Omicron induced by an adjuvanted subunit vaccine. Image Credit: solarseven / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
Four groups of male Rhesus macaques were used for the current study. In the first group, five macaques received immunization against RBD-Wu-AS03 at days 0 and 21, followed by a booster dose about six months later.
The second group received two doses of RBD-I53-50-Wu, and the third group had received two doses of HexaPro-I53-50. Both of RBD-I53-50-Wu and HexaPro-I53-50 were administered on days 0 and 21, alongside the AS03 adjuvant. These mice were then boosted with an I53-50 nanoparticle displaying RBD-Beta stabilized with Rpk9 mutations.
The final group consisted of unvaccinated controls. All animals were challenged with 2x106 units of the SARS-CoV-2 Omicron variant. The first group was challenged six weeks after the final dose, while the other two groups were tested about six months after the final booster.
Study findings
Vaccination with RBD-Wu-AS03 successfully elicited binding IgG titers against the spike protein of the SARS-CoV-2 wild-type, Omicron, and Beta strains by day 21. These titers increased by ten-fold after the second immunization but were reduced to pre-booster levels by six months.
The booster dose further improved humoral immune response, as evident by an increase in titers by 2.5- and four-fold against the wild-type and Omicron variants, respectively. Neutralizing antibody (nAb) responses were detected against the ancestral strain after the first dose, which increased by about 20-fold following the second dose.
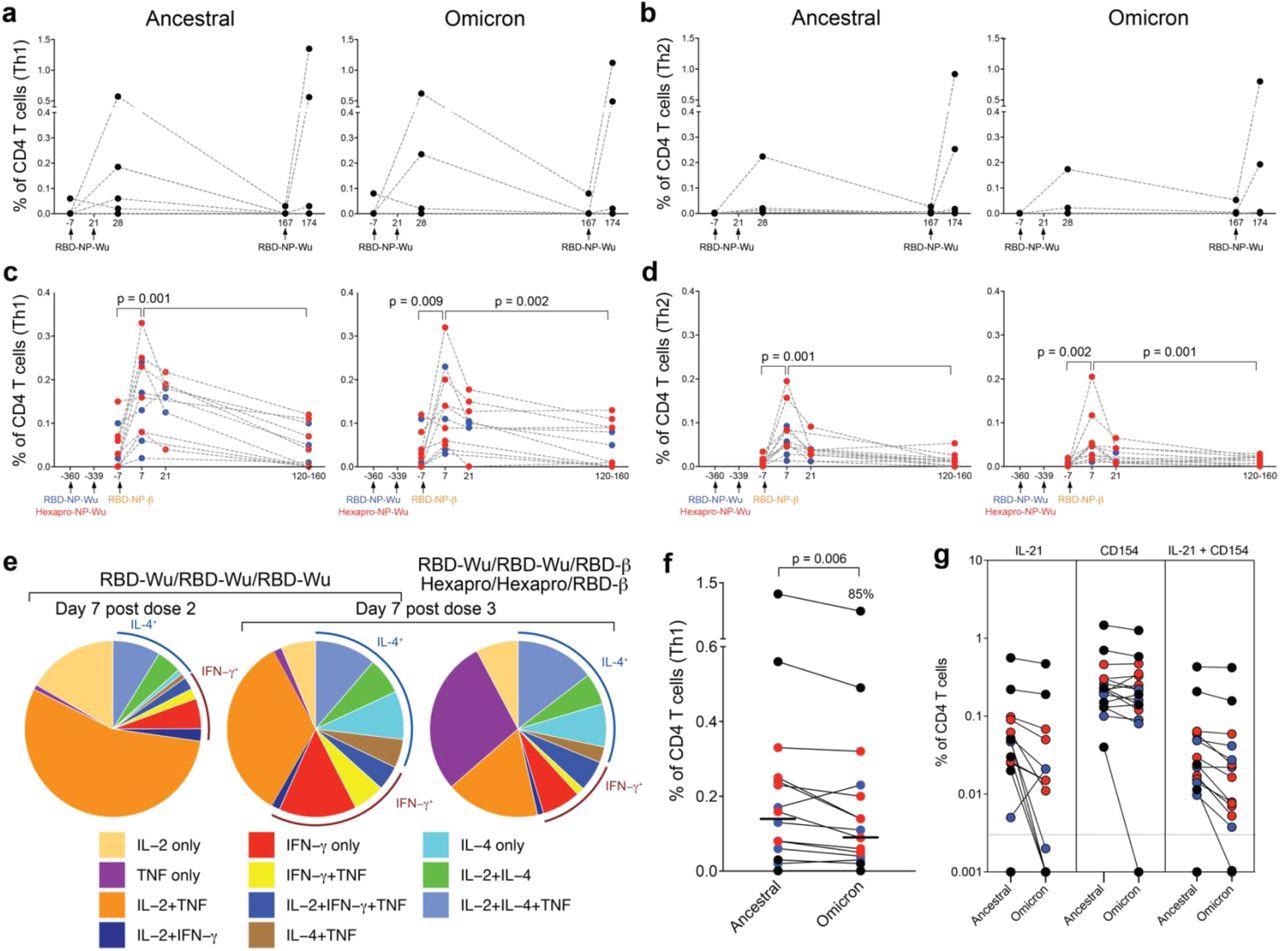
T cell responses induced by AS03-adjuvanted RBD-nanoparticle vaccination. a - b, Frequency of Spike-specific CD4 T cell responses against ancestral (left panel) and Omicron (right panel) strains in the RBD-Wu/RBD-Wu/RBD-Wu group. c – d, Frequency of Spike-specific CD4 T cell responses against ancestral (left panel) and Omicron (right panel) strains in the RBD-Wu/RBD-Wu/RBD-β (blue) and HexaPro/HexaPro/RBD-β (red) groups. CD4 T cells secreting IL-2, IFN-γ, or TNF are plotted as Th1-type responses (a, c) and IL-4-producing CD4 T cells are shown as Th2-type responses (b, d) The statistical differences between time points were determined using Wilcoxon matched-pairs signed rank test. e, Pie charts representing the proportions of RBD-specific CD4 T cells expressing one, two, or three cytokines as shown in the legend. f, Comparison of CD4 T cell frequencies between ancestral and Omicron viral strains measured on day 7 post final booster immunization. The statistical difference was determined using Wilcoxon matched-pairs signed rank test. The % value on top of Omicron represents the proportion of Omicron-specific responses relative to responses against the ancestral strain. g, Spike-specific IL-21+, CD154+, and CD154+IL-21+ CD4 T cell responses measured in blood on day 7 post final booster immunization. In all plots, each circle represents an animal, In f and g, black, blue, and red colors indicate RBD-Wu/RBD-Wu/RBD-Wu, RBD-Wu/RBD-Wu/RBD-β and HexaPro/HexaPro/RBD-β groups, respectively.
Notably, this immune response was not observed against the Omicron strain, as only a weak nAb response was detected. Following the booster dose, the response against both Omicron and the ancestral strain increased significantly.
In groups two and three, the booster dose of RBD-Beta elicited similar IgG responses to those seen in group one. This was a significant improvement, given the typical reduction in immune response over time. Neutralization activity was also still detectable prior to the booster and subsequently increased to significant levels following the final immunization.
As many previous studies have observed, antibody titers were significantly lower against the Omicron variant compared to the ancestral SARS-CoV-2 strain. Using a power-law decay model assuming decay rates decrease over time, the estimated half-life of binding IgG antibodies was shown to be very similar between different groups.
T-cell responses were measured using intracellular cytokine staining assays following the stimulation of peripheral blood mononuclear cells (PBMCs) with peptide pools spanning the spike proteins of the different variants. RBD-Wu-AS03 vaccination elicited both Th1 and Th2 CD4+ T-cell responses, which fell to baselines levels by six months before the booster dose raised them once again.
Similar responses were seen in groups two and three. These responses had decreased significantly five months post-booster but remained detectable.
Spike-specific B-cells were assessed using flow cytometry analysis of PBMCs with fluorescently-tagged spike protein of the different variants. To this end, a robust response was observed 21 days after the second vaccination with RBD-Wu-AS03. The third dose increased the frequency of spike-specific B cells by about ten-fold; however, the frequency decreased gradually and reached pre-immunization levels around six months.
Conclusions
The novel vaccine described in the current study offers effective protection against SARS-CoV-2, as it induced high levels of both humoral and cellular immune responses. Additionally, this vaccine was found to confer protection against the Omicron variant six weeks following the final booster. The current findings also demonstrate the durability of the induced immune response, especially with regards to neutralizing antibody titers.
As worries continue to be raised regarding the rapid waning of immunity provided by currently approved COVID-19 vaccines, the vaccine described here could be more effective in providing long-term immunity against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Arunachalam, P. S., Feng, Y., Ashraf, U., et al. (2022). Durable protection against SARS-CoV-2 Omicron induced by an adjuvanted subunit vaccine. bioRxiv. doi:10.1101/2022.03.18.484950. https://www.biorxiv.org/content/10.1101/2022.03.18.484950v1.
- Peer reviewed and published scientific report.
Arunachalam, Prabhu S., Yupeng Feng, Usama Ashraf, Mengyun Hu, Alexandra C. Walls, Venkata Viswanadh Edara, Veronika I. Zarnitsyna, et al. 2022. “Durable Protection against the SARS-CoV-2 Omicron Variant Is Induced by an Adjuvanted Subunit Vaccine.” Science Translational Medicine 14 (658). https://doi.org/10.1126/scitranslmed.abq4130. https://www.science.org/doi/10.1126/scitranslmed.abq4130.