Although effective vaccines providing short-term protection against coronavirus disease 2019 (COVID-19) were developed at a record-breaking speed, there remains significant vaccine hesitancy in many places around the world, even among educated people and medical personnel. A new study under consideration at the journal Trials and published on the preprint server Research Square* examines the reasons behind COVID-19 vaccine hesitancy emergency department (ED) patients when educated through appropriate vaccine messaging platforms.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
With almost one million people who have died due to COVID-19 in the United States alone in just over two years, vaccines have been embraced by public health authorities worldwide as a powerful method of reducing the risk of severe illness and hospitalization following infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, vaccine hesitancy poses a formidable barrier for at least 15% of eligible individuals according to responses to earlier surveys.
To overcome the limitations of online or telephonic surveys, the investigators in the current preprint surveyed patients in the ED, a universally available and often the only point of access to healthcare for one in five people in the U.S. This includes immigrants, homeless, poor, and uninsured people, as well as ordinary citizens. Since most of these are risk factors for adverse outcomes from COVID-19, broad-based participation would be provided in the exploration of factors leading to vaccine hesitancy.
Moreover, minorities receive a much greater share of primary healthcare through EDs, which makes these facilities an excellent starting point for vaccine delivery to the disadvantaged.
The Rapid Evaluation of COVID-19 Vaccination in Emergency Departments for Underserved Patients (REVVED UP) study, which was conducted by the authors of the current study, also surveyed ED patients from groups that were medically underserved at 15 locations.
The results of this study demonstrated that vaccine hesitancy was greater among those whose primary healthcare access point was the ED. Furthermore, this study revealed that the type of intervention needed in these groups to counter vaccine hesitancy was distinct from conventional community-based techniques used to encourage vaccination.
Study design
The current study is based on the idea that the ED is ideal as a healthcare access point for such populations in three different areas including fair vaccine distribution, achievement of population immunity, and disease prevention efforts.
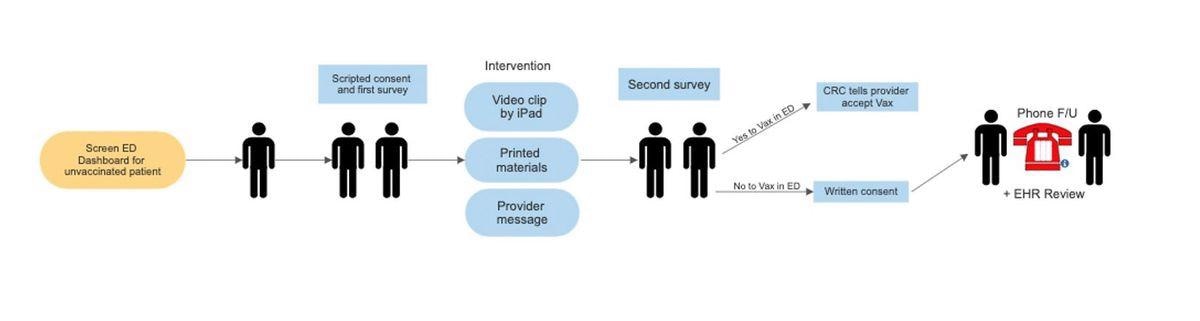
Intervention Blocks Study Flow
By conducting personal interviews with vaccine-hesitant patients whose primary healthcare is provided in the ED, the researchers hope to understand the fears driving vaccine hesitancy in this group. Using this knowledge, they developed customized messaging materials about the COVID-19 vaccine that were designed to allay these specific concerns.
The study is aimed at determining the extent to which these PROCOVAXED platforms will be successful in enhancing vaccine willingness in this group in terms of getting vaccinated during the ED visit or within 32 days of the ED visit.
The current study will use these platforms in seven EDs across four U.S. cities. Each site sees up to 250 patients a day, and patients will be randomized to have or not have this messaging delivered to them, depending on the week of the study. Patients who present to the ED during the weekdays and within a time span of six to ten hours were included in the study.
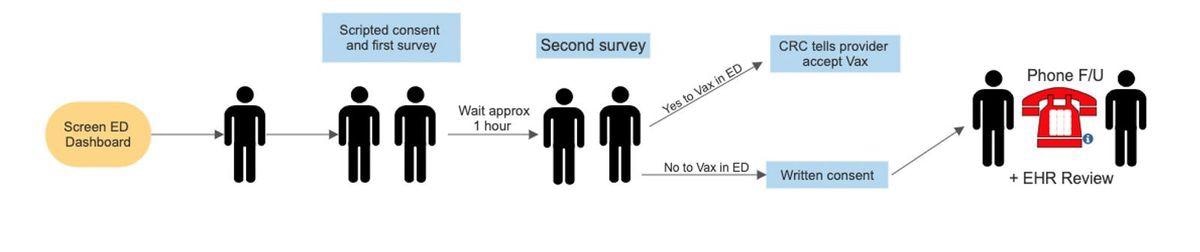
Control Period Blocks Study Flow
Patients will be presented with short videos, printed materials, or personal scripted messages delivered face to face. The videos last approximately four minutes and are based on the model of public service announcements. These videos will be delivered by physicians of African American origin, LatinX in English or Spanish, mixed-race, and White race.
The printed materials have the same format and words; however, the images follow the same aforementioned pattern. The face-to-face messaging is printed and spoken by an ED physician, nurse, or other practitioner involved in the care of the patient.
Permission to show the video will be first sought, followed by permission to hand over the flyer, regardless of whether the video is watched first. Once the flyer is given to the patient, the survey staff ask to be allowed to take a Vaccine Acceptance Survey in an hour, but the personal message is delivered during this interim period.
Enrolment for this trial began in December 2021 and is ongoing. Early termination of the study may occur if this messaging results in increased vaccine hesitancy or if there is such an improvement in vaccine acceptance seen even from early results that further non-intervention would not be ethically justified.
The baseline vaccine acceptance rate was estimated to be 15%, which is why the researchers are looking to enroll at least 1,300 patients in both arms together to detect an enhanced acceptance rate by 7% in the weeks during which PROCOVAXED is implemented.
Expected results
The ED is a vital point where acute care and public health interventions are offered to a large proportion of the U.S. population. Taking advantage of this, the researchers sought to devise a means whereby COVID-19 vaccine hesitancy could be mitigated and vaccine uptake enhanced in the same high-risk populations that are also the primary users of ED services.
The current study pivots on the assumption that specific messaging platforms directed to these ED patients from disadvantaged and vulnerable communities would improve their vaccine uptake. The messages will, however, reach the unvaccinated patients, thus allowing for further categorization of responses by subpopulation, which will help evolve further targeted messaging.
Moreover, the use of printed, spoken, and video messaging will help determine which method is most effective in different groups. The study is limited by the fact that during the period when it was being established, vaccine uptake increased to over 90%, thus making it difficult to obtain interviews with truly hesitant individuals and increasing the rate of interviews with vaccine-resistant people.
Conclusions
Our research may set a new paradigm for public health interventions to vulnerable populations, including messaging for other vaccinations like influenza, through the ED. The sheer number of ED visits across the country afford our research very high impact. If our intervention increases vaccine acceptance and uptake in 7% of vaccine hesitant patients, this could potentially lead to the delivery of tens of thousands of COVID-19 vaccines to people who would not otherwise get vaccinated.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Rodriguez, R., O’Laughlin, K., Eucker, S., et al. (2022). PROmotion of COvid-19 VA(X)ccination in the Emergency Department – PROCOVAXED: Study Protocol for a Cluster Randomized Controlled Trial. Research Square. doi:10.21203/rs.3.rs-1405763/v1. https://www.researchsquare.com/article/rs-1405763/v1.
- Peer reviewed and published scientific report.
Rodriguez, Robert M, Kelli N O’Laughlin, Stephanie A Eucker, Anna Marie Chang, Kristin L Rising, Graham Nichol, Alena Pauley, et al. 2022. “PROmotion of COvid-19 VA(X)Ccination in the Emergency Department—PROCOVAXED: Study Protocol for a Cluster Randomized Controlled Trial.” Trials 23 (1). https://doi.org/10.1186/s13063-022-06285-x. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-022-06285-x.