I am a surgeon-scientist dedicated to providing the latest approaches to treat male infertility and sexual medicine conditions, and contributing to innovative future treatments in my field. I have always had a passion for science and innovation. I was extremely fortunate to receive my training by pioneers in the field while at Weill Cornell Medicine in New York.
Since returning to one of the most innovative and scientifically productive urology departments in the world at the University of British Columbia, the environment has fostered key collaborations and infrastructure to develop an innovative interdisciplinary research program in male reproduction and sexual medicine.
Seeing patients every day who have problems that I cannot currently solve motivates me to think differently for ways that we can challenge current dogmas and develop technological solutions to improve treatments for these individuals.
Currently, around 15% of couples will struggle to conceive, with males being the contributing factor to over half of these cases. Why is therefore so important to find new treatment options for infertility? What benefits would this have not only for fertility but for men’s mental health also?
Struggling with infertility is very emotionally taxing for patients and couples. The ability to have children and raise a family is foundational in many individuals’ and couples’ beliefs, and when challenges are encountered it can be devastating and have negative consequences on wellbeing and work productivity among other areas of their lives. Developing novel therapies to offer the ability for couples with no current fertility options to potentially have children would be life-changing.
Image Credit: Pikovit/Shutterstock.com
The most severe form of male infertility, non-obstructive azoospermia (NOA), can only be helped in some cases through surgery and this procedure is only successful about half of the time. Why is this surgery not always successful and why do some individuals with NOA have no treatment options?
This surgery relies on the surgeon’s ability to find very rare areas of active sperm production among the billions of cells within the testicle. Even using a microscope, it is possible that we cannot find rare sperm that may exist; or, it is also possible that zero sperm are being produced. Therefore, in these patients, it is not possible to find and retrieve sperm.
It is among these groups, where zero sperm can be found, that we are focusing our efforts to develop technological solutions to either find rare sperm or, in the case of this study, to develop the technology to overcome deficits in sperm production for patients. By 3D printing cells in a controlled environment in the lab, we could potentially use additional growth factors and nutrients to encourage the stem cells to eventually differentiate and develop into sperm.
In your groundbreaking research, you have been able to 3D print human testicular cells. Can you tell us more about how you carried out your latest research and what you discovered?
Our research is based on the understanding that normal sperm production is a highly complex and coordinated event among roughly 20 cell types within the testicle that require multi-directional cell communication through sending molecules between them or being in physical contact with each other. These interactions are specifically coordinated in the seminiferous tubule within the human testis.
We thought 3D printing cytoarchitecture as close to this physical structure as possible would serve as the most advantageous paradigm for us to build the rest of our research on. A lot of technical development in our protocols were necessary to produce the results in this paper, and we are currently working to continually refine our methodology and improve the technology.

Image Credit: The University of British Columbia
You are now working to help “coach” these 3D-printed sperm cells into actually producing sperm. How are you doing this and what do you hope to see?
We are building an innovative scientific pipeline to develop this technology. By leveraging key collaborations across computational science & mathematics for analyses and algorithm development, clinical medicine, reproductive medicine, and biomedical engineering we have developed the ability to investigate the basis of normal human sperm production and deficiencies contributing to NOA.
We then eventually applied these findings to this 3D bioprinting platform which serves as both a functional model to help us perform more experiments and understand cell-to-cell communication and interactions, as well as a potential regenerative therapy platform.
Our approach requires development on two fronts. First, we are working to optimize the physical 3D printing to better position the cells and choose/develop bioinks to facilitate cell growth and development. This work is going on in our laboratory with help from collaborators such as Dr. Stephanie Willerth (University of Victoria Engineering & Axolotl Biotech).
Secondly, we need to understand cell needs and molecular signaling as the stem cells differentiate through various states as they progress to sperm. To accomplish this, we are using various modalities of single-cell sequencing to understand how cells are supporting the process of spermatogenesis, as well as the molecular events that are changing as stem cells differentiate through various cell states in spermatogenesis.
We are also comparing how these processes differ from cells derived among those with NOA where a problem in sperm production is occurring. Identifying these gaps may allow us to identify strategies to overcome these problems in a controlled laboratory setting. This is where we are working closely with Dr. Faraz Hach (Computational Science, University of British Columbia) and Dr. Geoffrey Schiebinger (Mathematics, University of British Columbia) to create and apply unique algorithms to understand the data and eventually apply it to our 3D bioprints where we could supplement the cells with unique growth factors at specific time points.
Are you hopeful that one day your technique will be used to help treat people living with untreatable forms of male infertility? What further steps need to happen before this can become a reality?
It is our dream to make this become a reality. We have a long road of scientific investigation, and technological development before we are able to make this platform ready for clinical use. Of course, these experiments are costly, so we will be working to secure additional grant funding, and philanthropy. Upon producing functional sperm in the future, appropriate safety data will be necessary before translation to the clinical space.
Your research, as well as producing these printed cells, has also helped to shed further light on the genetic mechanisms behind NOA. Can you tell us more about how you are doing this and what you have discovered so far?
We are using various modalities of single-cell sequencing to understand how cells are supporting the process of spermatogenesis, as well as the molecular events that are changing as stem cells differentiate through various cell states in spermatogenesis. We are also comparing how these processes differ from cells derived among those with NOA where a problem in sperm production is occurring.
Identifying these gaps may allow us to identify strategies to overcome these problems in a controlled laboratory setting. This is where we are working closely with Dr. Faraz Hach (Computational Science, University of British Columbia) and Dr. Geoffrey Schiebinger (Mathematics, University of British Columbia) to create and apply unique algorithms to understand the data.
So far, our data suggests that some of the supporting cells involved in coordinating and supporting stem cells to differentiate into sperm are not functioning properly. Thereby, correcting these supporting cells or supplementing additional growth factors and signaling molecules appear to be a potentially feasible strategy in the future. Based upon our data and the state of the literature, we hypothesize that the precise mechanisms are likely quite variable from patient to patient, and therefore a personalized medicine approach will likely be necessary moving forward.
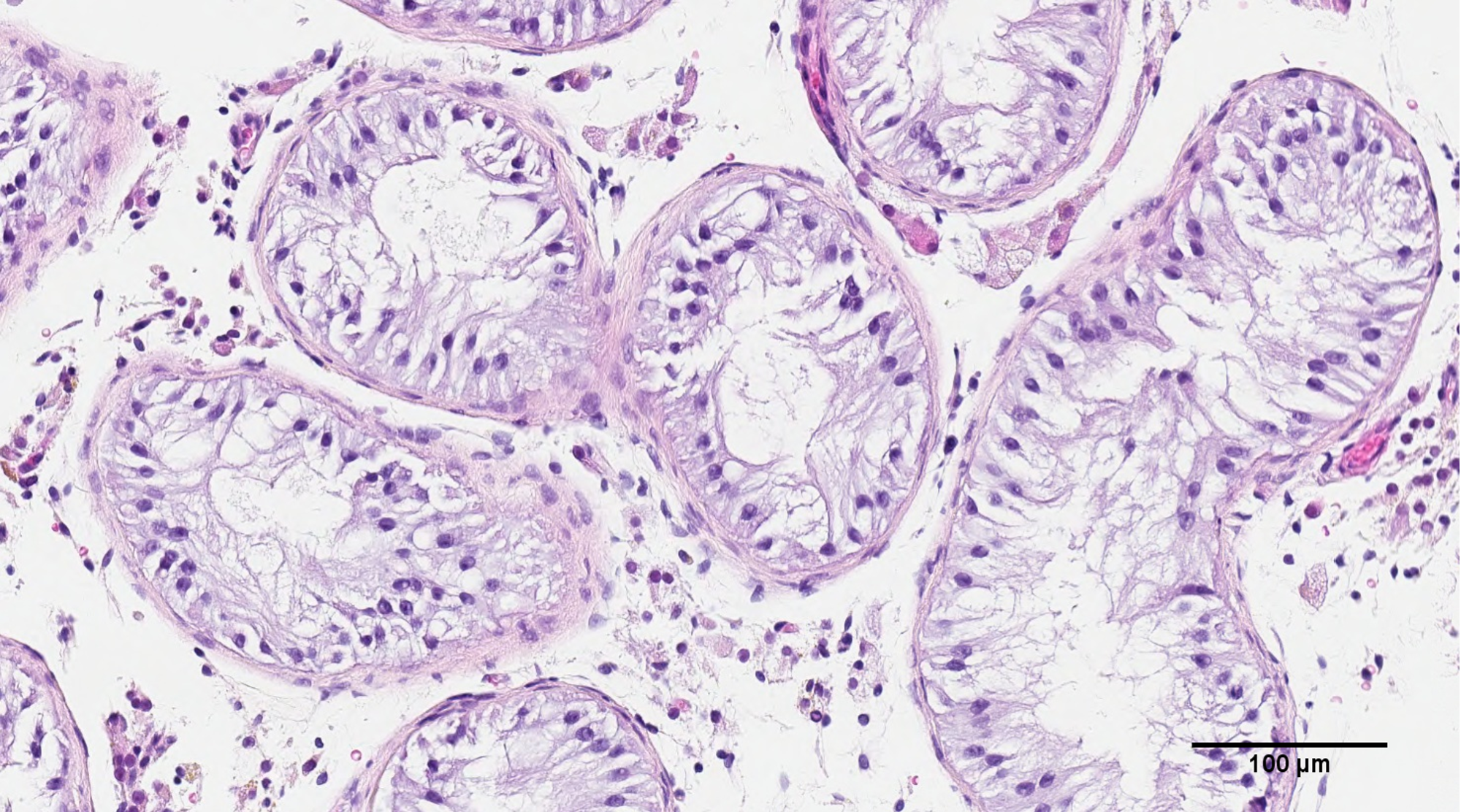
Image Credit: The University of British Columbia
As research continues, it is becoming more apparent that a ‘one-size-fits-all’ approach to treatment is not always the best option. Why are personalized options sometimes more beneficial? Do you believe that with continued research, we will see more precision medicine-based approaches being developed?
In medicine, diagnoses serve as a mechanism to categorize patients by which we can apply a systematic approach to evaluation and management. This is absolutely necessary and highly effective. However, we are progressively becoming more aware that each individual’s molecular physiology is unique and our cells function and respond ever so differently. As such, the intersection between disease heterogeneity and individual physiology means that we all likely have subtle differences in requirements for treatment and how we will respond to such therapies.
Therefore, the more granular we can understand individual disease and physiology, the more tailored a therapy we can develop and apply for that individual, and potentially provide the best chance of success. This, in various capacities, will be the direction of future medicine and is already being implemented in many instances.
Despite the devastating consequences of the COVID-19 pandemic, it has highlighted the incredible scientific advancements that can be made through collaboration. How important is collaboration to your research?
Our research program is highly interdisciplinary and truly relies on key collaborations to be innovative. We are grateful to be working with truly outstanding scientists within our lab (Meghan Robinson), Dr. Faraz Hach’s lab, Dr. Stephanie Willerth’s lab, Dr. Geoff Schiebinger’s lab on this project among others for our various other research directions (Dr. Hadi Mohammadi – Engineering, Dr. Hong Ma – Engineering, Dr. Nada Lallous – Urologic Biology, Dr. Christopher Ong – Urologic Biology, Dr. Hooman Sadri Ardekani – Reproductive Biology, Dr. Peter Schlegel – Male Infertility, Dr. Dolores Lamb – Reproductive Biology, Dr. Colin Collins - Genomics).
Technology and science are moving so quickly that being a master of all fields required to come up with the necessary solutions is challenging to say the least, and therefore collaborations are key, where each member of the research program contributes expertise and innovation to a very high level in their domain. Put all of these pieces together, and the product can be quite exciting.
What are the next steps for you and your research?
We are working to continue to understand the mechanisms of normal human spermatogenesis as well as those abnormalities contributing to failed sperm production (NOA). We are also working to fine-tune the bioink and 3D cytoarchitecture in our 3D bioprinting platform to better facilitate cell-cell interactions.
Where can readers find more information?
My clinical website is www.flanniganfertility.ca
My laboratory website is https://www.flanniganlab.com/
My YouTube Channel is: https://www.youtube.com/channel/UC7DtwVgWTyvO1cPH1P7SNpQ
My Instagram Account is: https://www.instagram.com/flannigan_fertility/
My Twitter Account is: https://twitter.com/RyanFlannigan00
My Facebook Account is: https://www.facebook.com/FlanniganFertility
About Dr. Ryan Flannigan
I am a surgeon-scientist and an Assistant Professor in the Department of Urologic Sciences at the University of British Columbia, with an Adjunct Assistant Professor position at Well Cornell Medicine, in New York. I have created and served as the director of the Male Reproduction & Sexual Medicine Research Program within the Department of Urologic Sciences, at the University of British Columbia.
In the first 4 years of my faculty position, I have attracted nearly 2 million in competitive research funding from provincial, national, and international societies, including the Canadian Institute of Health Research, Canadian Foundation for Innovation, Michael Smith Foundation for Health Research, New Frontiers Research Fund, Canadian Urologic Association Scholarship Foundation, American Society for Reproductive Medicine, Sexual Medicine Society of North America, Vancouver Coastal Health Research Institute. I am the lead author on the upcoming Canadian Urologic Association Azoospermia (infertility) guidelines.
I have initiated and directed a fellowship training program in male reproduction, sexual medicine, and microsurgery for urologists interested in subspecialty training in the clinic, operating room, and scientific laboratory.My clinical and surgical practice is subspecialized in male reproduction, sexual medicine, and microsurgery.