Various studies have reported the role of angiotensin-converting enzyme-2 (ACE-2) receptors as the point of entry for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This information makes the ACE-2 receptors a critical focus in the assessment of RAAS-mediated hypertension and COVID-19 severity.
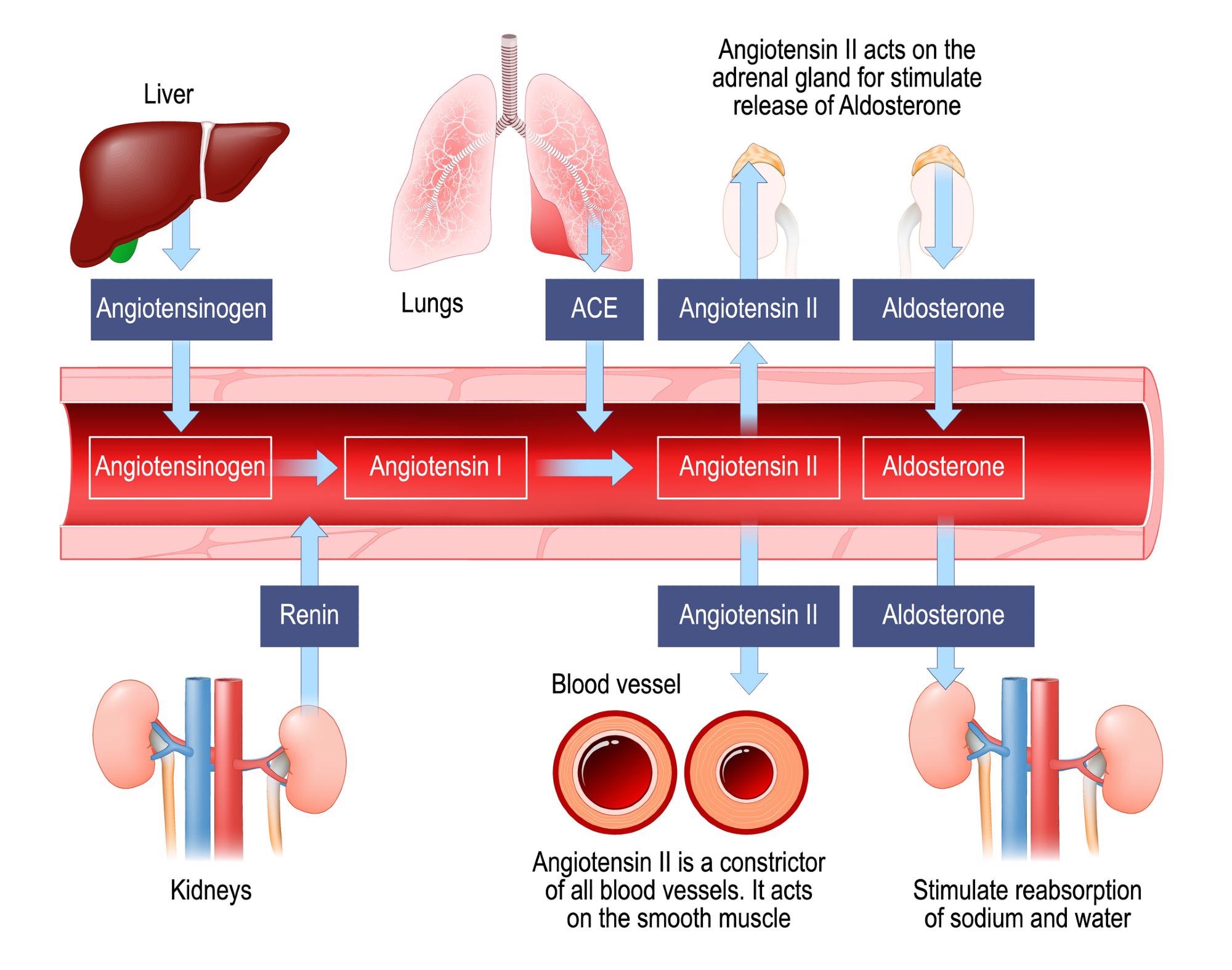 Renin–angiotensin–aldosterone system. Image Credit: Designua / Shutterstock
Renin–angiotensin–aldosterone system. Image Credit: Designua / Shutterstock

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the present study, researchers explored the physiology of risk factors related to the impact of COVID-19 on RAAS-targeted HT drugs and the interactions of hyperlipidemia (HL) with other risk factors associated with severe COVID-19.
The team collected data from electronic health records obtained from emergency and clinical health services and insurance records. The extracted records were classified into different sets based on several measures.
Out of the 1,000,000 randomly selected samples, approximately 997,140 were eligible for the study. However, the final study cohort included 269,536 COVID-19 patients plus an equal number of individuals who were not diagnosed with COVID-19, totaling 539,523 participants.
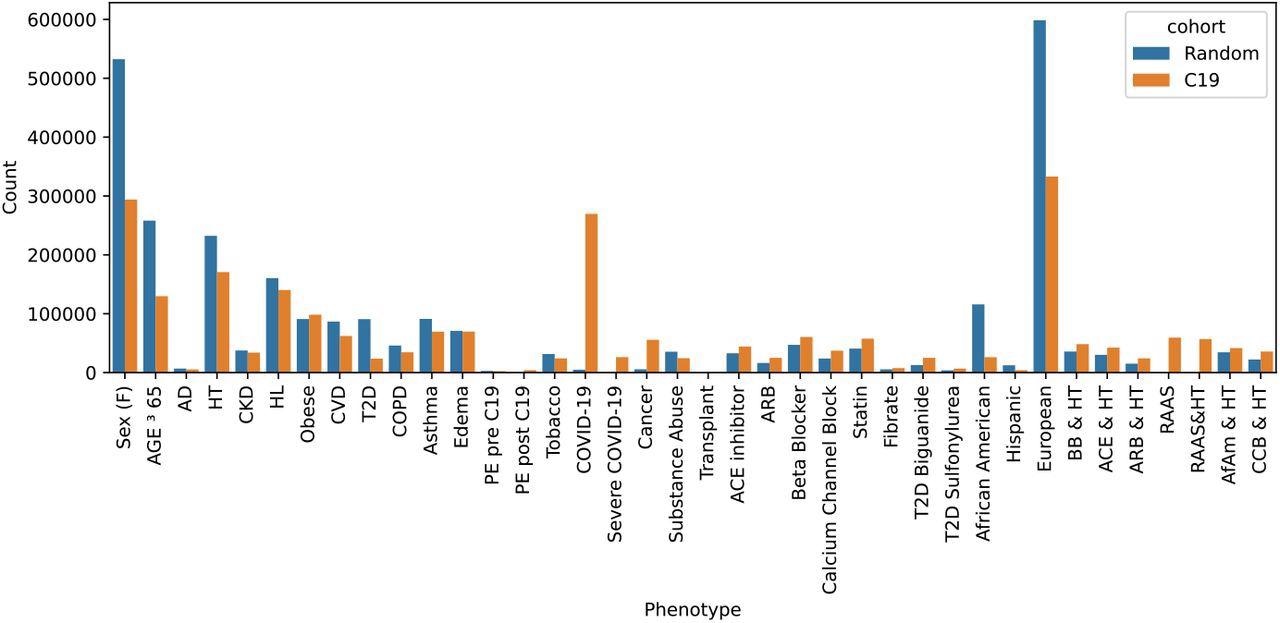
Two sample cohorts were drawn from the Explorys system: a random sample and all C19 patients plus an equal-sized randomly selected non-C19 control group. The total counts indicate the number after dropping missing entries. Abbreviations include Alzheimer’s disease (AD), hypertension (HT), chronic kidney disease (CKD), hyperlipidemia (HL), cardiovascular disease (CVD), type 2 diabetes (T2D), pulmonary edema (PE), beta-blocker (BB), calcium channel blocker (CCB), African Americans (AfAm).
The team evaluated the impact of the RAAS hypertension pathway on manifestations of severe COVID-19 by analyzing the interaction of HT with RAAS drugs, including ACE inhibitors and angiotensin receptor blockers (ARBs), and comparing these interactions with non-RAAS medications like beta-blockers and calcium channel blockers.
Redescription-based topological data analysis (RTDA) was performed using phenotypic combinations that could distinguish underlying biological processes and related pathways. The team applied computation homology to determine topological loops found among the pathways. The collections of deformed simplices found in these loops represented connected redescriptions. Such redescriptions were organized into homology groups to obtain more information about the complex disease processes. The team also applied persistent homology analysis to examine homology groups. The representative cycles were subsequently used to characterize relevant redescriptions as markers for homological cycles.
The team performed cumulant-based network analysis (CuNA) and extracted data on associations between RAAS drugs, COVID-19 manifestations, age, gender, and susceptibility to COVID-19 infection and severity. Homological cycles were selected to highlight inclusions that involved severe COVID-19 and three types of HT drugs, viz. ACE inhibitors, beta-blockers, and ARBs.
Results
The study results showed that among the two sample sets extracted, the random cohort comprised 997,140 individuals, the COVID-19-infected cohort comprised 269,536 patients, and the control group comprised 269,987 individuals.
The team found a strong association between HT and severe COVID-19. Additionally, distinctive metabolic syndrome features were observed among the study populations, including susceptibility to HT drugs, type 2 diabetes, and subsequent kidney failure. In these populations, calcium channel blockers were the primary choice of medicines for HT treatment, whereas RAAS drugs were not used often by severe COVID-19 patients.
The homological cycles indicated that HL posed a risk factor among patients diagnosed with severe COVID-19. In contrast, logistic regressions (LR) did not determine the first-order association between HL and COVID-19 severity. Also, LR predicted the presentation of severe COVID-19 only in the infected cohort treated with ARBs and ACE-inhibitors. This indicated that hyperlipidemia was not a critical risk factor for severe COVID-19 patients.
Moreover, the team found that hyperlipidemia substantially reduced the effect of other metabolic syndrome conditions on COVID-19 severity. Hyperlipidemia also lowered the impact of other factors, including being a male, being aged 65 years or above, or the presence of underlying conditions such as hypertension, pulmonary edema, and chronic kidney disease during the year of experiencing severe COVID-19, along with obesity, and type 2 diabetes. The authors noted that only the presentation of a cardiovascular disease aggravated COVID-19 severity.
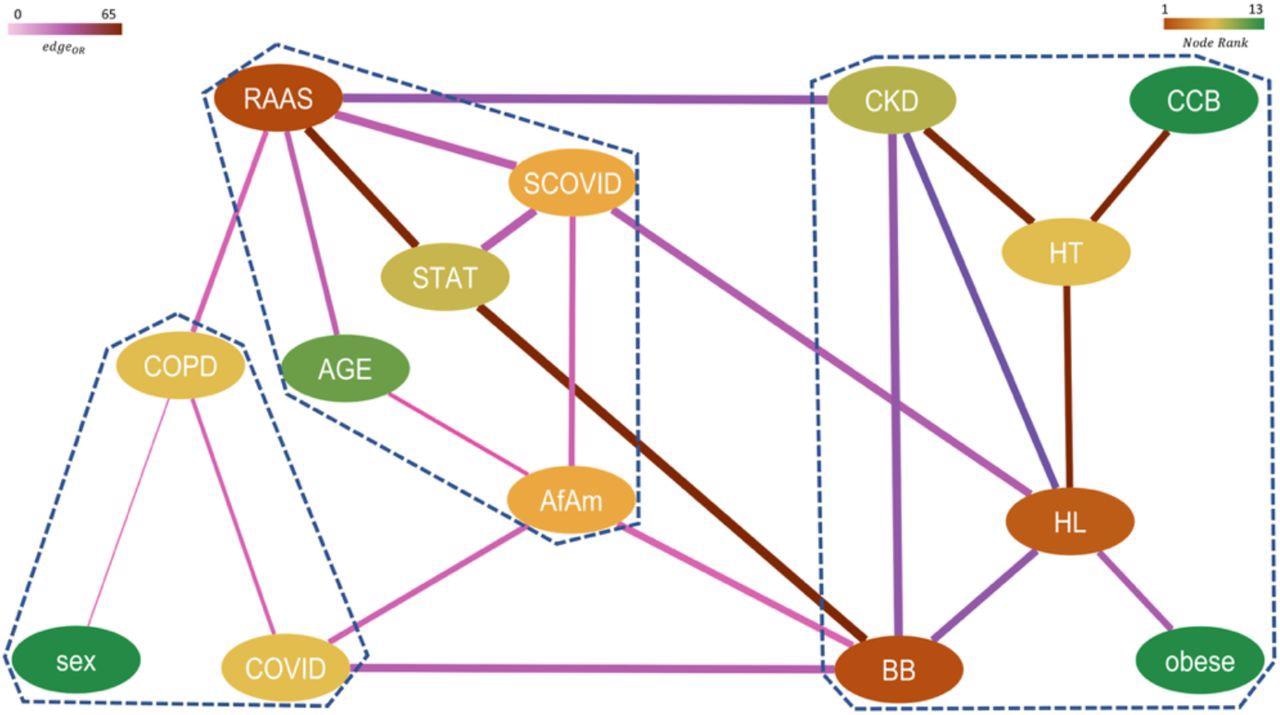
Network representing higher-order significant interactions between different features with nodes colored by their relative rank (gradient of brown to green corresponds to higher to lower rank) and edges colored by their respective pairwise ORs (gradient of light to dark corresponds to low to high OR) and edge width indicates the strength of the connection between them. The communities obtained from this network are marked by dashed lines.
Conclusion
The study findings showed that the RAAS complex played an important role in the exacerbation of chronic kidney disease in severe COVID-19 patients. The team also noted a trend that indicated that RAAS drugs suppressed the correlation of hypertension with severe COVID-19 more significantly than non-RAAS drugs. Furthermore, the researchers believe that this study highlighted the potential of topological data analysis in developing epidemiological studies that can efficiently target specific relationships to reveal novel connections that traditional analyses cannot fathom.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.