Various studies have reported that IC patients are highly susceptible to severe coronavirus disease 2019 (COVID-19) even after being fully vaccinated. Sotrovimab, a monoclonal antibody, has been found to neutralize SARS-CoV-2 and can thus serve as a treatment method for SARS-CoV-2-infected IC patients.
 Study: High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. Image Credit: Cryptographer / Shutterstock
Study: High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. Image Credit: Cryptographer / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
The researchers of the present study examined the alterations in SARS-CoV-2 after administration of sotrovimab in IC patients infected with SARS-CoV-2 Omicron BA.1 or BA.2 sublineages.
The study included Omicron-infected IC patients treated with sotrovimab in the inpatient and outpatient settings. The team performed a SARS-CoV-2 polymerase chain reaction (PCR) test at baseline, every week after the treatment started, and after hospital discharge until the PCR cycle threshold (Ct) value was 30 or higher. The researchers also sequenced the baseline and follow-up samples with a Ct value of less than 30.
In the present study, the team defined successful sequencing as a minimum of 90% of the viral genome having at least 30-fold coverage. Immunoglobulin G (IgG) spike antibody titer was subsequently evaluated 30 days after initiation of the treatment.
Results
The study results demonstrated that among the 47 patients eligible for the study, 51% were males and the median age of the cohort was 63 years. Among these, 66% of the patients had a history of an organ transplant, 28% were treated with B-cell depleting therapy, and 0.1% received other immunosuppressives for an auto-immune disease.
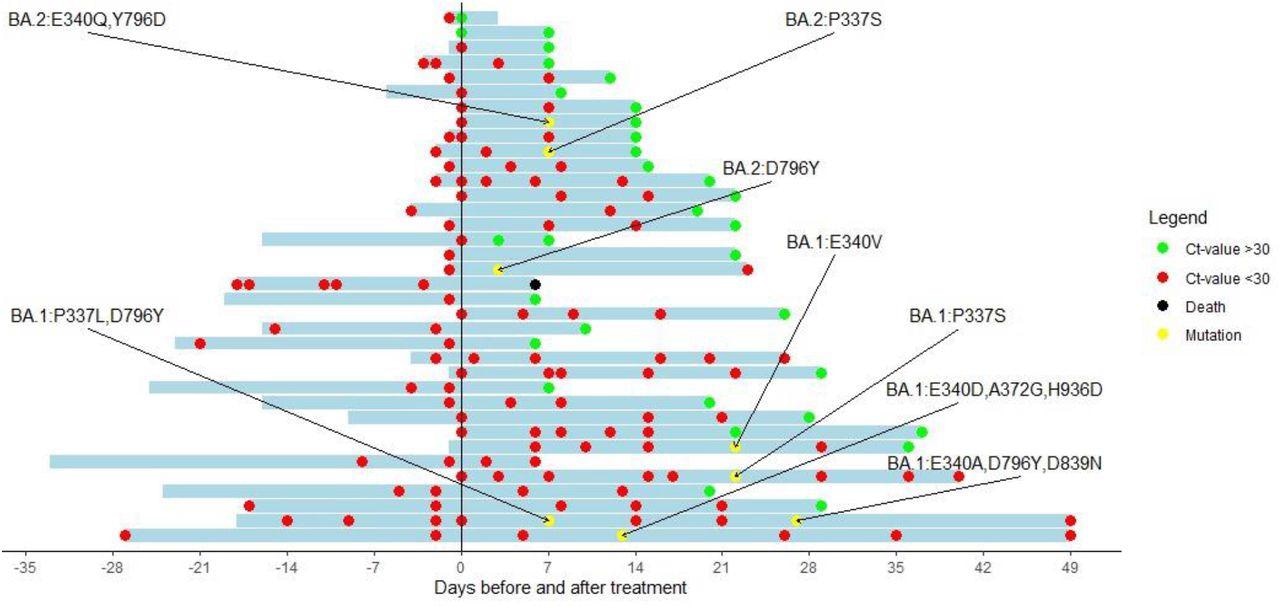
Cyclic threshold value of patients treated with sotrovimab with a baseline RT-PCR and at least one follow-up RT-PCR available. The y-axis indicates the day of sotrovimab infusion. Red dots represent a Ct-value <30. Green dots represent a Ct-value of >30. Black dots represent the death of a patient. Yellow dots represent a sequence showing new Spike mutations compared with the baseline sequence.
All the patients were vaccinated with at least two messenger ribonucleic acid (mRNA) vaccine doses, while 24 patients had received three or more doses. Despite this, 18 patients had no spike antibodies, while 25 had very low levels of the same. In the present study, 66% of the patients were treated with sotrovimab.
The median time required to attain a Ct value of 30 or more was 15 days. However, four patients showed low Ct values 29, 36, 49, and 49 days after sotrovimab treatment. The researchers successfully sequenced RNA samples from 25 and seven patients infected with the BA.1 and BA.2 variant, respectively, while 17 samples were successfully sequenced for the baseline and follow-up RNA samples. Among these 17 samples, six showed key spike mutations at positions 337 and 340 that have been known to induce resistance to sotrovimab in vitro. Of the six patients, four and two patients were infected by BA.1 and BA.2, respectively.
In a patient infected with the BA.2 variant, a D796Y mutation was detected. However, its influence on sotrovimab at a half-maximal inhibitory concentration (IC50) was unknown. The four BA.1-infected patients were found to have mutations at position 337 or 340 of the SARS-CoV-2 spike protein.
Conclusion
The study findings showed that the evolving viral resistance could explain the failure of the sotrovimab treatment in most IC patients. The researchers believe that since IC patients are unable to clear viral load even after antiviral treatment, these patients can harbor the novel SARS-CoV-2 variants. Further research is needed to ascertain the efficacy of antivirals in IC patients and the potential of combination therapies in lowering the risk of viral resistance.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. Sammy Huygens, Bas Oude Munnink, Arvind Gharbharan, Marion Koopmans, Bart Rijnders, medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.04.06.22273503, https://www.medrxiv.org/content/10.1101/2022.04.06.22273503v1
- Peer reviewed and published scientific report.
Huygens, Sammy, Bas Oude Munnink, Arvind Gharbharan, Marion Koopmans, and Bart Rijnders. 2022. “Sotrovimab Resistance and Viral Persistence after Treatment of Immunocompromised Patients Infected with the SARS-CoV-2 Omicron Variant.” Clinical Infectious Diseases, July. https://doi.org/10.1093/cid/ciac601. https://academic.oup.com/cid/article/76/3/e507/6648554.