
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Protection from COVID-19, which arises due to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been provided by several vaccines, such as those produced by Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273). These two mRNA-based vaccines are typically administered in a two-dose regimen, with three to four weeks between each dose.
Recent reports have suggested that the duration of vaccine efficacy is about 250 days post-vaccination with 70% efficacy. Studying the efficacy of COVID-19 vaccines can help understand waning immunity and determine the need for additional vaccine doses.
About the study
The current study involved a matched case-control (1:30) design that consisted of 62,555 individuals infected with SARS-CoV-2, which was determined by a positive reverse-transcription polymerase chain reaction (RT-PCR) assay, between January 1, 2021, and February 20, 2022, in Qatar.
The two groups included in the current study were those receiving normal care, which consisted of 62,871 individuals, and those admitted to the ICU, which included 2,102 individuals.
Notably, 64.7% of the normal care group were males, with 73% of those admitted to the ICU also male. Patients who received either the BNT162b2 (10 or 30 µg), mRNA-1273 17277, or AstraZeneca ChAdOx1 vaccine were included in the study.
The interval between the first two doses was 21 days for the BNT162b2 vaccine and 28 days for the mRNA-1273 vaccine. For a third booster dose, the median interval was 246 days. The same type of vaccine was administered in the booster. In the case of mRNA-1273, a half dose was used for booster vaccination.
The presence of certain comorbidities including diabetes, hypertension, cancer, obesity, asthma/COPD, cardiovascular, cerebrovascular, rheumatological, kidney, neurological disease, hematological, immunity-related, and liver diseases were recorded. Ethnicity and gender were not considered confounders.
Study findings
Vaccination against COVID-19 was found to be 59% effective in preventing ICU admission between the first and second dose. In this regard, vaccine efficacy increased to 89% after the second dose and remained at 91% between four to six after the second dose.
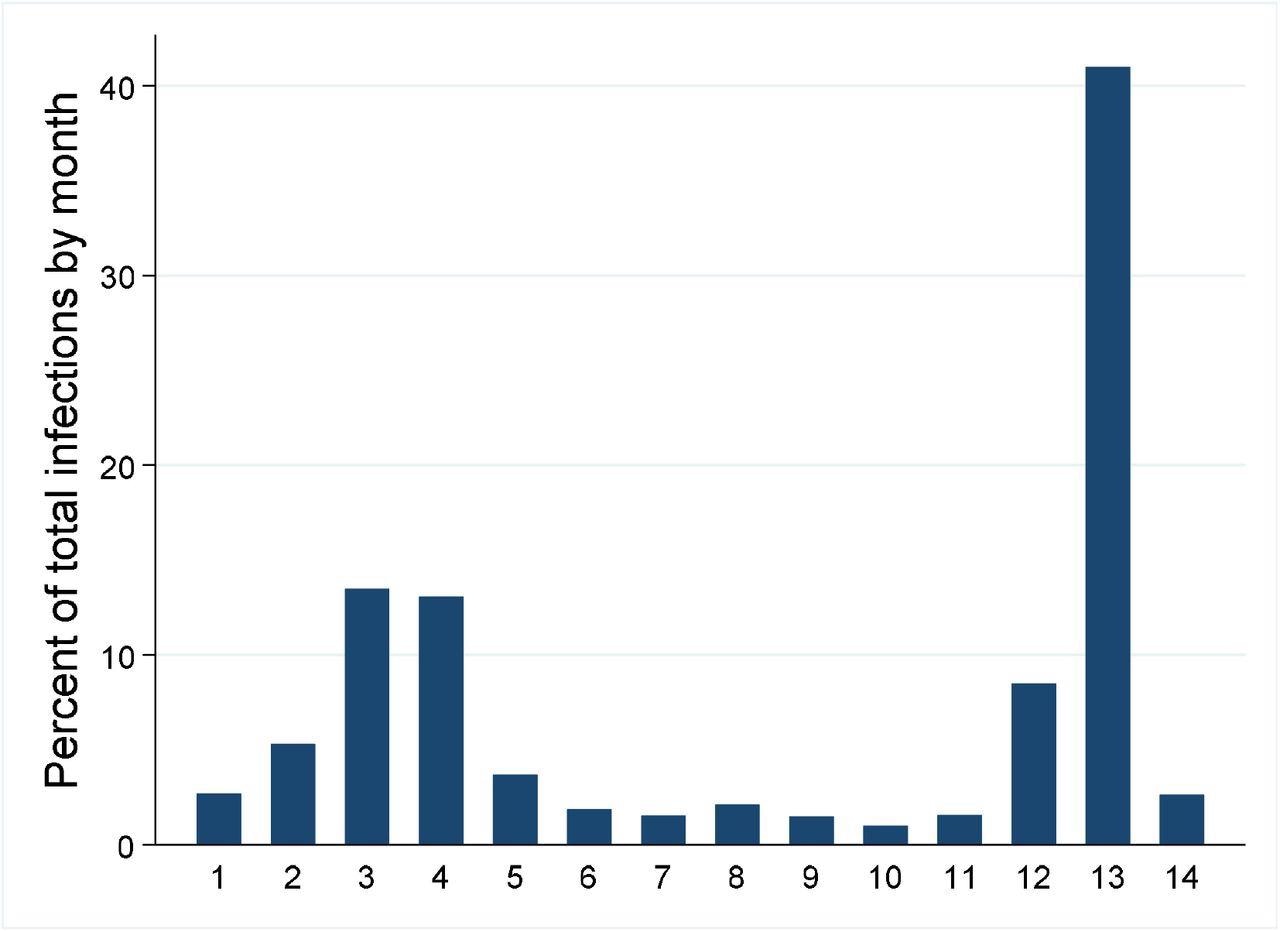
The two major waves are depicted over the 14 month study period (1-12 are Jan – Dec 2021 and 13-14 are Jan-Feb 2022).
Between six to nine months after complete vaccination, vaccine effectiveness was slightly reduced to 90%. Furthermore, between nine and 12 months after the second dose, vaccine effectiveness against ICU admission was 94%. After the booster dose, vaccine effectiveness in this regard increased to 95%.
No significant difference was observed in vaccine effectiveness before and after excluding participants who received the AstraZeneca vaccine. Interestingly, patients’ age was an essential factor in determining whether ICU admission was required.
The number needed to vaccinate (NNV) value increased from nine at age 60 to 90 at age 30 between nine and twelve months after receiving the second vaccine dose. This indicated that a lower NNV value is associated with a greater risk of ICU admission in vulnerable groups.
Conclusions
The study findings demonstrate that mRNA vaccination remains up to 94% effective for up to 12 months after the second dose in preventing ICU admission, which is in contrast to the waning immunity observed in previous studies. Importantly, the current study avoided several biases found in the earlier reports by considering the entire cohort in Qatar. However, additional research is needed to determine vaccine efficacy against ICU admission after 12 months.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bansal, D., Abdulmajeed, J., Yassin, E., et al. (2022) Duration of COVID-19 mRNA Vaccine Effectiveness against Severe Disease. medRxiv. doi:10.1101/2022.04.12.22273745. https://www.medrxiv.org/content/10.1101/2022.04.12.22273745v1
- Peer reviewed and published scientific report.
Bansal, Devendra, Jazeel Abdulmajeed, Maha H. M. A. Al-Shamali, Soha S. A. Albayat, Sayed M. Himatt, Farhan S. Cyprian, Tawanda Chivese, et al. 2022. “Duration of COVID-19 MRNA Vaccine Effectiveness against Severe Disease.” Vaccines 10 (7): 1036. https://doi.org/10.3390/vaccines10071036. https://www.mdpi.com/2076-393X/10/7/1036.