Following the emergence of SARS-CoV-2 in late December 2019, the coronavirus disease 2019 (COVID-19) pandemic has affected global health systems. Shortly after SARS-CoV-2 emergence, numerous COVID-19 vaccines were developed centered on the SARS-CoV-2 original (Wuhan) strain spike (S) sequence.
SARS-CoV-2, like other ribonucleic acid (RNA) viruses, is vulnerable to mutational changes over time. As a result of the widespread dissemination of the COVID-19 pandemic, adaptive changes are more likely to emerge, potentially leading to selective viral benefits such as improved attachment to human cells or immunological escape from neutralizing antibodies. Mutations in the SARS-CoV-2 S receptor-binding domain (RBD) are concerning since the virus gains host cell entry through this region. Furthermore, the altered amino acid residues in the mutants can affect SARS-CoV-2 neutralization by vaccine or natural infection-induced antibody responses.
About the study
The present study aimed to discover more about immune responses to naturally occurring SARS-CoV-2 infections versus COVID-19 vaccination for the most severe mutant virus types. The researchers evaluated long (up to one year)- and short (<2 months)-term antibody and T cell responses in blood samples from completely COVID-19 vaccinated subjects and priorly SARS-CoV-2-positive tested individuals to the viral original and mutant sequences, with specific attention to the SARS-CoV-2 Delta (B.1.617.2) variant. Individuals were deemed fully COVID-19 vaccinated if there had been two weeks since their last shot of either a messenger RNA (mRNA)-based (mRNA-1273, BNT162b2) or vector-based (ChAdOx1 nCoV-19) vaccine.
The scientists assessed the anti-SARS-CoV-2 S and anti-nucleocapsid (N) protein antibody titers in the participants. The team determined the antibody-mediated average SARS-CoV-2 neutralization as the hindrance of angiotensin-converting enzyme 2 (ACE2) adherence to wild type (wt) and Delta RBD. Flow cytometric intracellular cytokine staining was used to evaluate SARS-CoV-2 S protein-specific CD4+ T cell responses following stimulation with peptide pools from the SARS-CoV-2 Delta, Gamma, Beta, Alpha, or wt mutant S proteins.
Results
The study results demonstrated that considering serum antibody binding to wild type (wt) and mutant virus proteins, all individuals vaccinated against SARS-CoV-2 had significant humoral immune responses. High anti-SARS-CoV-2 S protein RBD antibody titers were present in 23 COVID-19 vaccinees. Antibody-facilitated average SARS-CoV-2 neutralization was 2.2 times lower in the RBD of the SARS-CoV-2 Delta mutant than in the wt.
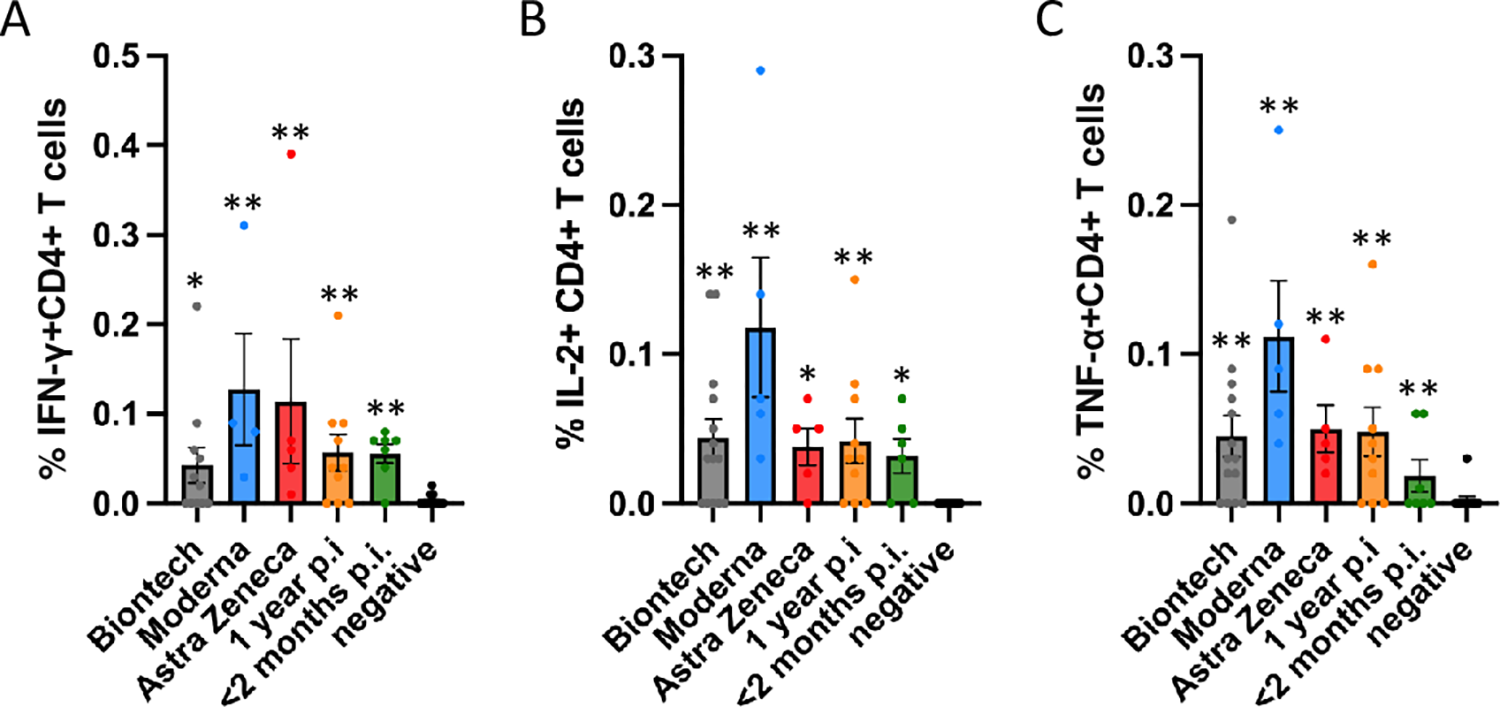 Antigen-specific CD4+ T cell responses elicited by the Miltenyi wt S protein peptide pool after vaccination with BioNtech (grey), Moderna (blue) or AstraZeneca (red), or one year (orange) or < 2 months (dark green) after naturally occurring infection with SARS-CoV2. In comparison, samples of healthy control subjects, who tested negative for SARS-CoV2-antibodies, are shown on the right of each panel. The bar graphs show frequencies of (A) interferon-γ (IFN-γ), (B) interleukin-2 (IL-2)- and (C) tumor necrosis factor-α (TNF-α)-positive CD4+ T cells. The figure shows results of n = 13 independent individuals after vaccination with BioNtech, n = 5 Moderna, n = 5 AstraZeneca, and n = 10 independent individuals one year and n = 7 subjects < 2 months after natural infection with standard errors of the mean (SEM). For comparison, 13 negative healthy controls were included. Significance levels were tested by Kruskal-Wallis test for non-parametric values. *p < 0.05, **p < 0.01 vs. healthy controls.
Antigen-specific CD4+ T cell responses elicited by the Miltenyi wt S protein peptide pool after vaccination with BioNtech (grey), Moderna (blue) or AstraZeneca (red), or one year (orange) or < 2 months (dark green) after naturally occurring infection with SARS-CoV2. In comparison, samples of healthy control subjects, who tested negative for SARS-CoV2-antibodies, are shown on the right of each panel. The bar graphs show frequencies of (A) interferon-γ (IFN-γ), (B) interleukin-2 (IL-2)- and (C) tumor necrosis factor-α (TNF-α)-positive CD4+ T cells. The figure shows results of n = 13 independent individuals after vaccination with BioNtech, n = 5 Moderna, n = 5 AstraZeneca, and n = 10 independent individuals one year and n = 7 subjects < 2 months after natural infection with standard errors of the mean (SEM). For comparison, 13 negative healthy controls were included. Significance levels were tested by Kruskal-Wallis test for non-parametric values. *p < 0.05, **p < 0.01 vs. healthy controls.
In more than 90% of COVID-19 vaccinees, SARS-CoV-2 neutralizing titers (NT50) remained considerably above the level correlated with immune protection in recent vaccination studies. SARS-CoV-2 neutralization ability was declined in many participants after COVID-19 and those who had received one dose of the vaccination. One year after natural SARS-CoV-2 infection, mean specific antibody titers were lower than after COVID-19 vaccination, indicated by the 1.65-fold lower ACE2 binding to the Delta mutant RBD versus the wt strain.
Many SARS-CoV-2 strains showed reduced cross-neutralization, which might affect COVID-19 vaccination effectiveness. The authors observed that Omicron RBD binding was decreased compared to the Delta variant.
The researchers discovered robust CD4+ T cell responses to SARS-CoV-2 mutant (Delta and Gamma) or wt S proteins. However, no particular CD8+ T cell response was seen, which might be because the 15-meric peptide pool used was insufficient to excite CD8+ T cells. Further, the authors found no drastic variation between the two-dose vaccinated study volunteers in cytokine production of tumor necrosis factor α (TNF-α), interleukin 2 (IL-2), or interferon γ (IFN-γ). Compared to vaccinated subjects, T cell responses to SARS-CoV-2 mutant or wt S proteins were substantially lower following SARS-CoV-2 infection.
Conclusions
According to the authors, this was the first work that carefully compared inhibition experiments that utilized labeled SARS-CoV-2 mutant versus wt RBD and ACE2 coated plates with those in which ACE2 was labeled and put in solution to mutant against wt RBD-coated plates.
The study findings depicted that the SARS-CoV-2 Delta mutant had lower antibody neutralization than the viral wt strain, as demonstrated in the new inhibitory assessment with a finger-prick blood drop. Strong CD4 T cell responses were found against SARS-CoV-2 mutant and wt strains, including the Delta (B.1.617.2) variant, in fully vaccinated subjects. However, CD4 T cell responses were somewhat weaker one year after natural SARS-CoV-2 infection.
Overall, these data imply that immunological responses following SARS-CoV-2 vaccination were more significant than those following naturally occurring COVID-19, highlighting the necessity for the vaccine to combat the SARS-CoV-2 pandemic. Furthermore, the present findings showed that using a combination of rapid production of recombinant proteins in mammalian cell systems and adoptive T cell assays, immunological responses to SARS-CoV-2 variants could be investigated within three months of their emergence.
Immune reactivity to future SARS-CoV-2 variants of concern and present viral mutants will allow vaccination methods to be adjusted to give population protection against new SARS-CoV-2 lineages of concern and to track the novel mutants spread. In light of the worldwide mutant spread, these findings suggest that additional COVID-19 vaccination should be undertaken following spontaneous SARS-CoV-2 infection. Moreover, the present paper describes a complementary diagnostic panel that could measure immunological reactivity to COVID-19 vaccines.