SARS-CoV-2 binds with the angiotensin-converting enzyme 2 (ACE2) of the host for initiating viral entry based on the transmembrane serine protease 2 (TMPRSS2) expression and S cleavage. In the NTD of SARS-CoV-2 spike, over 20 mutations have been identified, but very few have been characterized. Therefore, the impact of the mutations on viral infectivity and vaccine immunogenicity is unclear.
The authors of the present study had previously shown that the Delta S and Kappa S demonstrated high S1/S2 cleavage efficacy compared to the 614G WT (wild-type) strain and the infectivity of the SARS-CoV-2 strain correlated with the efficiency of S cleavage.
About the study
In this new study, researchers assessed the importance of NTD in SARS-CoV-2 S cleavage and fusion of the virus to host cells leading to subsequent SARS-CoV-2 infections.
The team constructed a panel of chimeric S that comprised either the Kappa NTD or Delta NTD in with their corresponding parental strain backgrounds. Subsequently, pseudoviruses (PV) expressing S of several variants of concern (VOC) were used for transducing the TMPRSS2-expressing 293T cells and parental 293T cells to assess the efficacy of SARS-CoV-2 invasion. Additionally, the difference in the fusogenicity of the Delta and Kappa strains was evaluated.
As an extended analysis, the WT S bearing either the Delta NTD or Kappa NTD was expressed. Further, the Delta NTD was introduced into the Omicron BA.1 and BA.2 sublineages to determine whether the Delta NTD could increase Omicron fusogenicity. In addition, the impact of BA.2 NTD on BA.1 fusogenicity and the converse were assessed.
The contribution of NTD in S cleavage was assessed in purified PVs by western blot analysis. Further, the dependence of SARS-CoV-2 on TMPRSS2 for host invasion was assessed by pretreating A549- ACE2/TMPRSS2 cells or airway organoids with either E64D (a cathepsin inhibitor) or camostat (a TMPRSS2 inhibitor). The alteration in ACE2 accessibility in the chimeric S was evaluated by transducing parental 293T cells with abolished TMPRSS2 expression and overexpression of ACE2.
The contribution of Delta NTD mutations in increasing viral infectivity was assessed in cultured human airway epithelial cells (Calu3) cells treated with PVs expressing S of different VOCs and reversion of amino acid clusters (156G, 142D, del158R, and del157F) to the WT Wuhan-1 strain.
To assess the effect of reversion of mutations on the vaccine sensitivity, the PVs were used for transducing HeLa-ACE2 cells with serial dilution of serum samples obtained from individuals doubly vaccinated with the BNT162b2 vaccine. In addition, viral neutralization assays were performed.
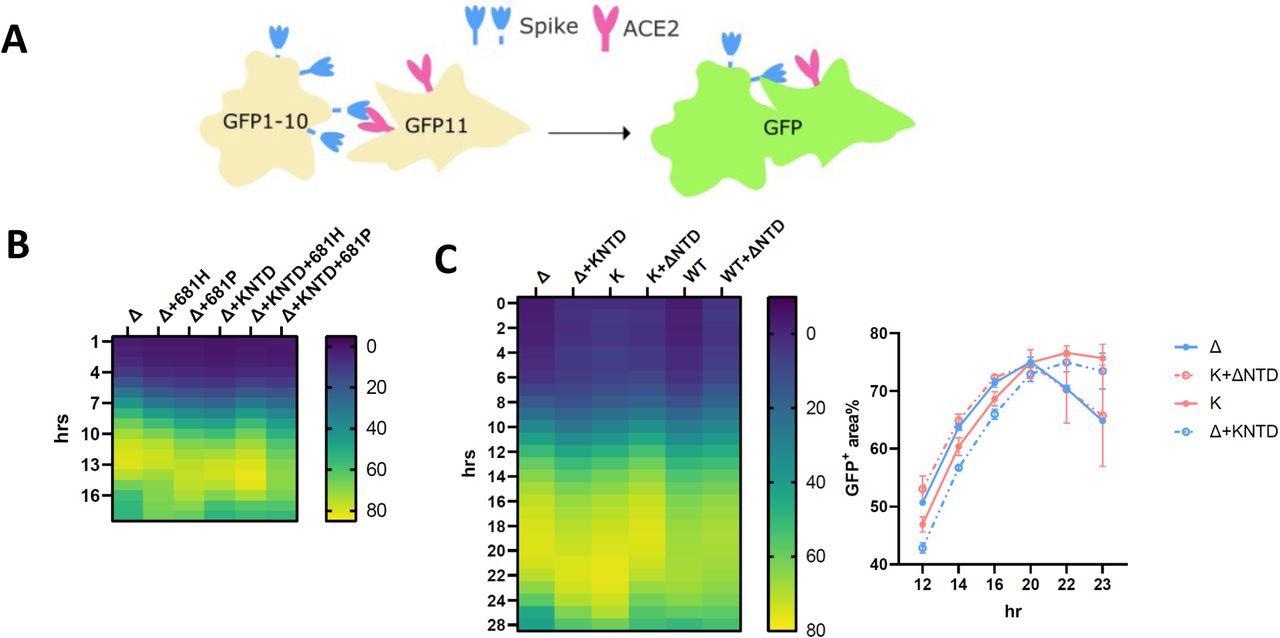
The SARS-CoV-2 Delta NTD increases the fusion kinetics of Kappa and WT spikes. (A): A schematic diagram showing the split GFP system for spike-ACE2 mediated cell fusion. (B): 681R or 681H is required for the enhanced fusogenicity in Delta and its chimera bearing Kappa NTD. (C): The fused Delta NTD in Kappa and WT increased the fusion kinetics of their counterparts, respectively. The line graphs on the right show the percentage of the positive GFP area at 12, 14, 16, 20, 22 and 23 hr post transfection. The data showing the SEM at each point were averaged from two experiments. The heatmap at each time point shows the mean of the GFP positive area over the field of view from two experiments.
Results
In accordance with the team’s previous findings of the association of increased S cleavage with increased fusogenicity and infectivity, enhanced TMPRSS2-mediated S1/S2 cleavage and increased infectivity were observed among the Kappa and Delta chimeras in the airway organoids and the Calu3 cells. In addition, Kappa S was prone to SARS-CoV-2 S1 shedding with a higher S2/S1 ratio in comparison to the WT strain, whereas Delta S demonstrated increased stability. In addition, Delta showed the highest efficiency of TMPRSS2-mediated SARS-CoV-2 entry and fusogenicity. Interestingly, increased entry efficiency of the Kappa chimeric S was observed, indicating that the Delta NTD enabled Kappa entry into the host by utilizing TMPRSS2.
In contrast, Omicron sublineages bearing the Delta NTD did not demonstrate any increase in fusogenicity, indicating that the Delta NTD could not increase the TMPRSS2 use efficiency of Omicron. In the extended analysis, the Delta NTD and the Kappa NTD enhanced S cleavage in the WT chimeras and thereby increasing the WT infectivity in the Calu3 cells.
In the pretreated cells and airway organoids, the half-maximal inhibitory concentration (IC50) value of camostat was two-fold higher for Delta compared to Kappa, while E46D showed negligible effects on SARS-CoV-2. Delta showed increased resistance to camostat and therefore demonstrated higher TMPRSS2 utilization efficiency for initiating virus entry.
On ACE2 overexpression, a 30-fold and a four-fold increase in Delta and Kappa efficiency, respectively, were observed. Of note, the Kappa chimeric S bearing Delta NTD demonstrated a similar degree of increase in sensitivity as Delta. On the other hand, reduced dependence on ACE2 was observed in the Delta chimera bearing Kappa NTD. This indicated the Delta NTD modulated the TMPRSS2-mediated S cleavage and allosterically regulated RBD for increased ACE2 utilization efficiency
Each reverted mutant showed reduced infectivity in Calu3 cells, with the highest (three-fold) reduction observed after reinserting 157R/158R. Of note, the reduction in infectivity was observed only in Calu3 cells and not in Hela-ACE2 cells, indicative of the SARS-CoV-2 specificity that was conferred by the NTD.
In the neutralization assays, the presence of G156E and D142G and the repair of the 157F/158R deletion increased the SARS-CoV-2 neutralization sensitivity by two folds, underpinning the importance of NTD for evasion of the host immune responses and infectivity of SARS-CoV-2.
In the fusion assays, on fusing the Kappa NTD into Delta, the fusion phenotype shifted to Kappa from Delta, with retardation of kinetics. On the other hand, a rapidly fusing Delta phenotype was observed on swapping the Delta NTD by Kappa. In addition, a faster fusion phenotype was observed on fusing the Delta NTD into the WT backbone. This indicated that the Delta NTD could improve the fusion kinetics of the WT and Kappa strains.
Overall, the study findings highlighted the variant-specific allosteric modulation of SARS-CoV-2 S cleavage and infectivity by NTD. The increase in S cleavage efficiency warranted a cognate NTD since the S bearing only receptor-binding domain (RBD) mutations could not be cleaved efficiently.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
SARS-CoV-2 spike N-Terminal Domain modulates TMPRSS2-dependent viral 2 entry and fusogenicity. Bo Meng, Rawlings Datir, Jinwook Choi, CITIID-NIHR BioResource COVID-19 Collaboration, John Bradley, Kenneth GC Smith, Joo Hyeon Lee, Ravindra K. Gupta, bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.07.491004, https://www.biorxiv.org/content/10.1101/2022.05.07.491004v2
- Peer reviewed and published scientific report.
Meng, Bo, Rawlings Datir, Jinwook Choi, Stephen Baker, Gordon Dougan, Christoph Hess, Nathalie Kingston, et al. 2022. “SARS-CoV-2 Spike N-Terminal Domain Modulates TMPRSS2-Dependent Viral Entry and Fusogenicity.” Cell Reports 40 (7). https://doi.org/10.1016/j.celrep.2022.111220. https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01037-3.