The antibodies induced after coronavirus disease 2019 (COVID-19) vaccination or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have a reduced capacity to cross-neutralize variants of concern (VOCs). However, antibodies are not the sole mediators of immunity.
 Study: Long-Lasting T Cell Responses in BNT162b2 COVID-19 mRNA Vaccinees and COVID-19 Convalescent Patients. Image Credit: Corona Borealis Studio / Shutterstock
Study: Long-Lasting T Cell Responses in BNT162b2 COVID-19 mRNA Vaccinees and COVID-19 Convalescent Patients. Image Credit: Corona Borealis Studio / Shutterstock
Cell-mediated immunity
T cells are the mediators of cell-based immunity. They play an essential role in immune protection, recovery from an acute infection, and long-lasting immune memory.
The antigen-presenting cells (APCs) present several short peptides of viral proteins to T cells. The T-cell responses are stimulated by these different short peptides. Therefore, the T-cell responses are not sensitive to viral protein mutations like the antibody responses.
The CD8+ cytotoxic T lymphocytes (CTLs) identify and destroy virus-infected cells. The CD4+ T helper (Th) cells coordinate and enhance CTL responses. They also stimulate antibody production by B cells.
Previous studies have shown that SARS-CoV-2 infection stimulates robust memory CD4+ and CD8+ T-cell responses. This cell-mediated immunity may provide long-lasting protection against reinfections.
Assessing cell-mediated immunity
This study analyzes the longevity of SARS-CoV-2 spike-specific antibody, and cell-mediated immunity in BNT162b2 vaccinated health care workers and COVID-19 patients.
This study included 23 fully vaccinated health care workers (two doses of BNT162b2 vaccine), 15 COVID-19 patients, and 13 individuals with no previous SARS-CoV-2 infection or COVID-19 vaccination. Blood samples were collected from the vaccinated individuals six weeks, three months, and six months after the first vaccine dose; the convalescent COVID-19 patients at 18 to 45 days (mean 33 days) after SARS-CoV-2 infection.
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood and activated with peptides from the whole SARS-CoV-2 spike protein. The T cell subtypes within the PBMC population were characterized using flow cytometry.
The expression of different cytokines was measured using RT-qPCR. In addition, the levels of cytokines and other molecules secreted by the cells were detected using Luminex. Anti-SARS-CoV-2 antibodies were measured using enzyme immunoassay (EIA). The activation-induced marker (AIM) assay was optimized before analyzing the samples.
PBMCs were stimulated with the wild-type SARS-CoV-2 Wuhan virus spike peptide pool. A 48h stimulation and measurement of IFN-γ and IL-2 mRNA levels were selected for all analyses. Tetanus toxoid was used as a positive control. Both IFN-γ and IL-2 are Th1-type cytokines.
T-cell responses
SARS-CoV-2 spike-specific CD4+ cell responses were detected in all COVID-19 patients and vaccinated individuals 6 weeks, 3 months, and 6 months after the first vaccine dose.
CD8+ cell responses were detected in 80% of COVID-19 patients and 70% of vaccinated individuals 6 weeks post first dose; in 67% of vaccinated individuals 3 months post first dose; and in 53% of vaccinated individuals 6 months post first dose. These T-cell responses were similar in the COVID-19 patients and vaccinated individuals.
The T-cell responses against VOCs were assessed by stimulating the PBMCs with peptide pools from spike proteins of Alpha, Beta, Gamma, and Delta variants. CD4+ cell responses against all VOCs tested were detected in over 71% of vaccinated individuals and over 75% of COVID-19 patients.
CD8+ T-cell responses against all VOCs tested were detected in over 50% of vaccinated individuals and over 75% of COVID-19 patients. However, significant differences were observed between wild-type and Gamma variant 6 weeks post-vaccination and wild-type and Beta variant 6 months post-vaccination.
COVID-19 patients had stronger CD4+ and CD8+ responses compared to vaccinated individuals. There was no decrease in cell-mediated immunity against VOCs after 6 months.
The expression of IFN-γ and IL-2 mRNA was detected in 93% and 100% of vaccinated individuals after stimulation with the wild-type and Delta variant spike peptide pool. The mRNA levels were higher compared to mRNA levels in PBMCs collected from unvaccinated and uninfected individuals. The IFN-γ and IL-2 mRNA expression were similar between COVID-19 patients and vaccinated individuals.
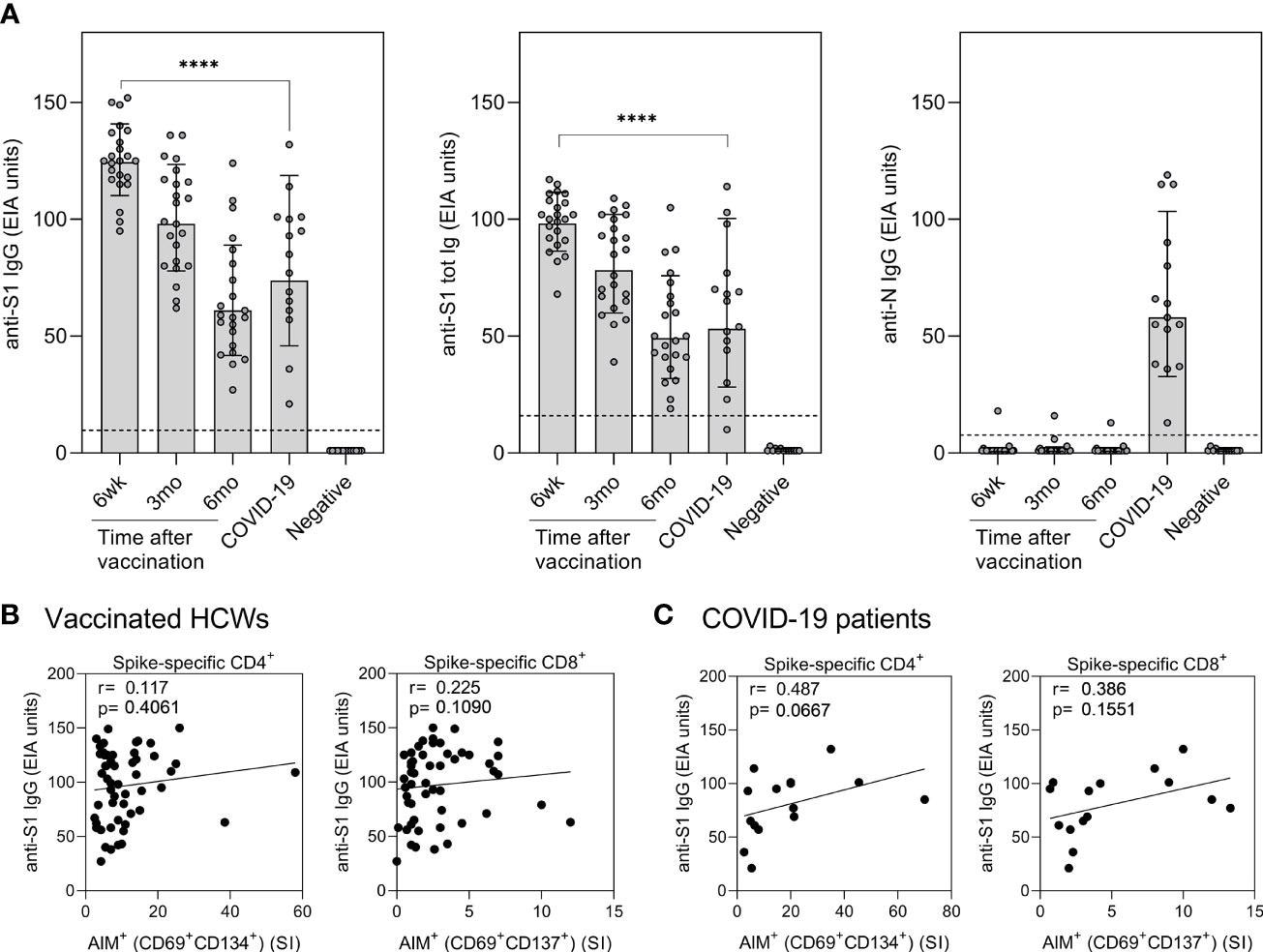
Correlation of humoral immunity to cell-mediated immunity after BNT162b2 vaccination n = 23 and SARS-CoV-2 infection. (A) Anti-SARS-CoV-2 S1-specific IgG, S1 total Ig, and N protein-specific IgG antibody responses were measured from samples collected at 6 weeks, 3 months, and 6 months after vaccination n = 23 or 1 month after PCR confirmed SARS-CoV-2 infection (n=15). Serum antibody levels of vaccinated and infected individuals were compared with negative controls (n=13) that had not received any COVID-19 vaccines or suffered from a previous SARS-CoV-2 infection. Bars represent the geometric mean titers. The cut-off values for a positive test result are shown with dotted line Statistical analysis was performed with Mann Whitney U-test for comparison of 6wk vaccinee samples with COVID-19 patient samples. ****p<0.0001. (B,C) The correlation of anti-SARS-CoV-2 S1 IgG antibody levels with SARS-CoV-2 (wt) spike specific CD4+ and CD8+ T cell responses in (B) vaccinated HCWs (samples collected 6 week, 3 months, and 6 months after vaccination, n= 52) and (C) COVID-19 patients (samples collected 1 month post-onset of symptoms, n=15). Correlation was analyzed with the Spearman’s correlation and Spearman’s r is indicated in the figures.
After stimulation of PBMCs from vaccinated individuals with wild-type and Delta variant spike peptide pools, IFN-γ and IL-2 protein levels increased significantly. The levels of IFN-γ and IL-2 were higher in vaccinated individuals and COVID-19 patients than in the unvaccinated uninfected individuals. IFN-γ and IL-2 levels did not decrease 6 months post-vaccination. Moreover, stimulation with wild-type or Delta variant peptide pools produced equivalent IFN-γ and IL-2 levels.
There was a high correlation between T cell activation and production of IFN-γ and IL-2 cytokines.
All vaccinated individuals produced spike-specific anti-SARS-CoV-2 antibodies six weeks after the first vaccine dose. The levels of antibodies were higher than those of hospitalized COVID-19 patients. In the vaccinated individuals, antibody levels decreased gradually.
The levels of anti-SARS-CoV-2 antibodies in vaccinated individuals did not correlate with CD4+ or CD8+ T-cell responses. However, in COVID-19 patients, the spike-specific anti-SARS-CoV-2 antibodies increased with increasing CD4+ and CD8+ T-cell responses.
Limitations of the study
This study cryopreserved the PBMCs until analysis. This may decrease cell viability, and some memory cells may be lost in the process. The number of participants assessed in this study was relatively low. Only BNT162b2 vaccinated individuals were studied. This study did not include vaccinated individuals over 60 years of age. The AIM protocol did not test for longer incubation times. Longer 15-mer peptide pools were used for stimulation instead of 9-to 10-mer peptides. This may underestimate the SARS-CoV-2-specific CD8+ cells.
Conclusion
This study shows that even if SARS-CoV-2 spike protein-specific antibodies decline with time after COVID-19 vaccination or natural infection, the cell-mediated immunity is retained to a great extent and is not sensitive to mutations in the viral spike protein.