The immunization of healthcare workers (HCWs) against CoV disease 2019 (COVID-19) remains critical given the present SARS-CoV-2 infection dynamics and the fast spread of viral variants of concern (VOC), particularly Omicron. COVID-19 vaccination can significantly decrease the severity of the SARS-CoV-2 infection and its potential transmission. These effects are crucial in the healthcare sector to avoid COVID-19-linked personnel shortages and maintain public healthcare capacity. However, there is inadequate information on the elements that affect humoral immunity against SARS-CoV-2.
Earlier works on anti-SARS-CoV-2-spike (S) humoral antibodies in HCWs had small study populations, short observation time, and did not address the quality of life, demographic characteristics, or capacity to work. In addition, there is a scarcity of similar large-scale COVID-19 seroprevalence data from real-life situations, notably among HCWs.
About the study
The present cross-sectional research evaluated the seroprevalence of anti-SARS-CoV-2-S immunoglobulin Gs (IgGs) in German HCWs following COVID-19 or its vaccination and the factors impacting it. The data depicted in this investigation was part of the prospective CoVacSer study that assessed COVID-19 immunity using serial blood samples and quality of life and capacity to work surveys in medical personnel following SARS-CoV-2 antigen exposure. The data collection for the present study was conducted from September 29 to November 12, 2021, coinciding with the fourth COVID-19 wave in Germany.
All the study subjects were enrolled in the investigation following the submission of the signed consent form. The final research cohort consisted of 1,750 study volunteers aged ≥18 years with polymerase chain reaction (PCR)-confirmed COVID-19 or at least one dose of SARS-CoV-2 vaccination and was employed in the healthcare field.
Serum blood samples for determining anti-SARS-CoV-2-S IgG were procured coupled with pseudonymized CoVacSer research surveys, including physical condition, personal risk factors, demographic data, and work ability index (WAI) and World Health Organization quality of life (WHOQOLBREF) questionnaires. Anti-SARS-CoV-2-S IgG titers were quantified using SERION enzyme-linked immunosorbent assay (ELISA) agile SARS-CoV-2 IgG. This test was selected since it was better than similar SARS-CoV-2 IgG ELISA techniques, exhibiting higher neutralization titer correlations.
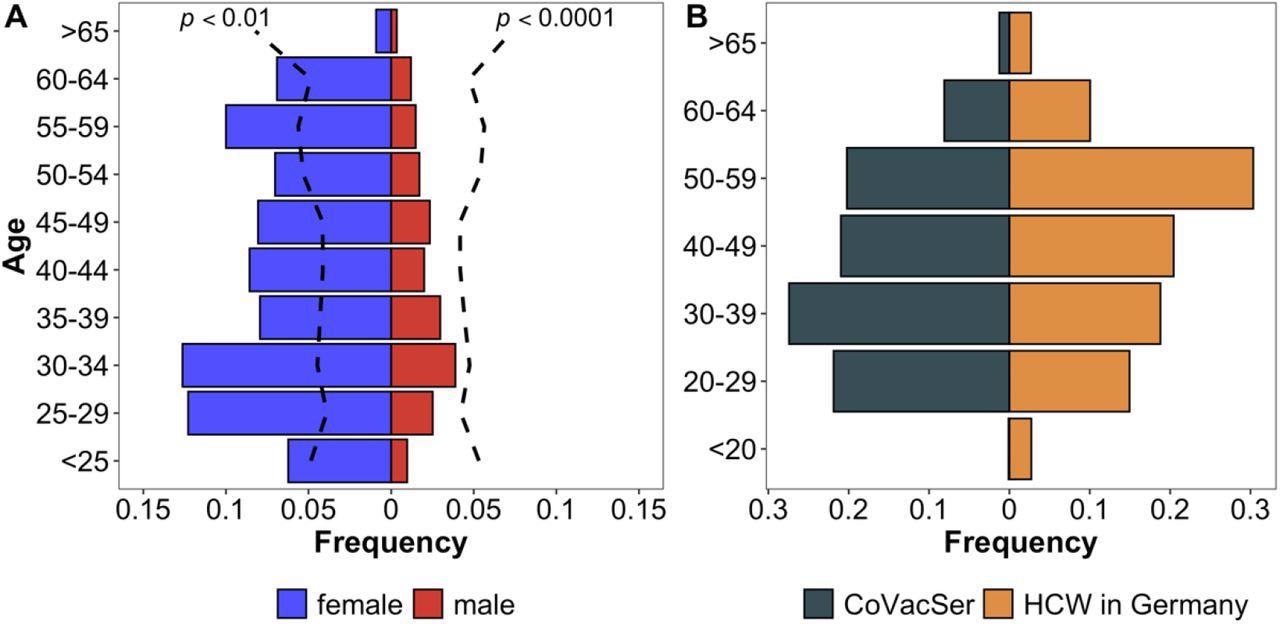
Characterization of study population compared to the German general public and HCWs in Germany Comparison of CoVacSer study population to reference populations. Fig. 2A portrays the enrolled HCW study population (portrayed in gender-separated blue and red bars, n = 1,750) in comparison to the demographic composition of the German general public considering gender and age (black broken line, Kolmogorov-Smirnov-Test) as of December 31, 2020. Fig. 2B compares the age structure in 10-year categories as a percentage of respondents included in the study (blue bars) with the total number of HCWs in Germany (red bars).
Results and discussions
The study results indicated that anti-SARS-CoV-2-S levels were detected in COVID-19 convalescent, SARS-CoV-2-vaccinated, and hybrid immunized HCWs, showing at least a moderate viral neutralizing potential. Mean anti-SARS-CoV-2-S IgG levels rose exponentially as the number of COVID-19 vaccines increased, i.e., 92.2, 140.9, and 1,144.3 BAU/ml after one, two, and three doses, respectively.
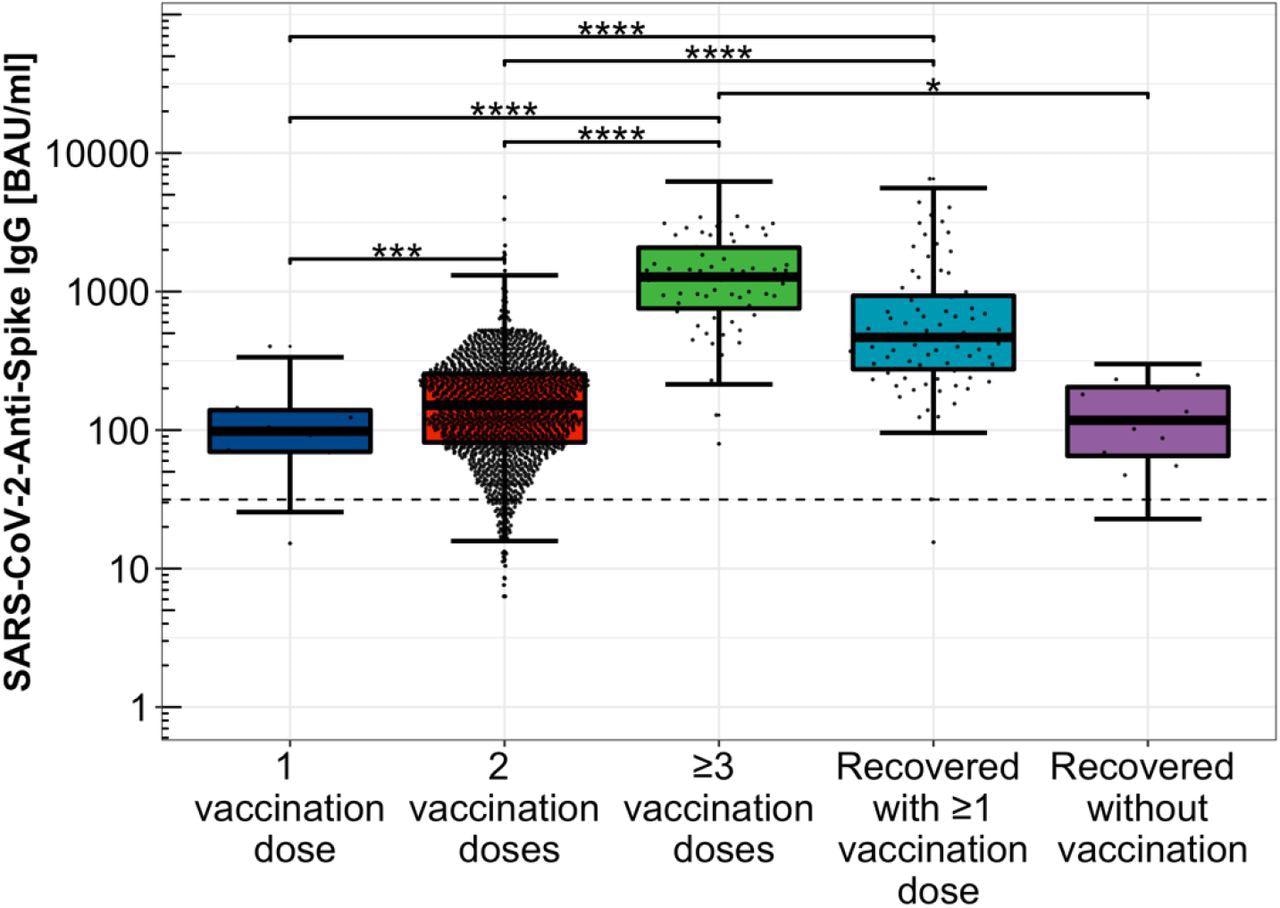
Distribution of Anti-SARS-CoV-2-Spike IgG levels depending on immunization scheme. Distribution of Anti-SARS-CoV-2-Spike IgG titers among single, double, and threefold COVID-19 vaccinated participants, only COVID-19 convalescent study participants as well as hybrid immunized participants including SARS-CoV-2 infection convalescence and COVID-19 vaccination, logarithmically scaled.
Subjects with hybrid SARS-CoV-2 immunization, i.e., history of COVID-19 and its vaccination, showed considerably greater antibody titers (525.4 BAU/ml) than those with just viral infection (105.7 BAU/ml). This inference supported the significance of the SARS-CoV-2 vaccination as a supplement to adequate humoral protection against COVID-19 post-viral infection. Further, antibody levels were considerably lower in the two-dose vaccinated group than in the hybrid immunized cohort.
Gender, age group, employment field, body mass index (BMI), smoking, immune deficiency, dependency on medical treatment, health status, subjective usefulness of life, contact with COVID-19 patients, vaccination concept, and time since the last SARS-CoV-2 immunizing event were all found to be linked to anti-SARS-CoV-2-S IgG levels using the lasso regression model. In addition, subjects with immune deficiencies displayed a trend toward reduced, yet not statically relevant, attenuation of the humoral immunity to SARS-CoV-2 infection or COVID-19 vaccination. Anti-SARS-CoV-2-S IgG titers dropped substantially with time following the receipt of the second dose vaccination, demonstrating a diminishing humoral immune response upon the baseline COVID-19 vaccination.
Further, increasing age and smoking had statistically relevant correlations to lower anti-S antibody titers versus respective comparison cohorts. Notably, the authors failed to illustrate the importance of the variables that lower anti-SARS-CoV-2-S IgG concentrations since there were no threshold ranges for IgG levels that safeguard against SARS-CoV-2 infection or severe disease.
Conclusions
According to the study findings, medical professionals who had been SARS-CoV-2 vaccinated or recovered from the viral infection showed a predominantly robust humoral immune response, with declining antibody levels with time. The humoral immunity against COVID-19 was hampered by higher age and smoking. Moreover, this lowered immune response was significant since SARS-CoV-2 patients with these risk characteristics were known to have a higher risk of severe COVID-19.
Overall, the present cross-sectional research reflected the need for the COVID-19 vaccine as a preventative intervention, particularly among at-risk HCWs who were highly exposed to SARS-CoV-2. According to the study findings, further research into the temporal pattern of anti-SARS-CoV-2-S IgG levels, and the impact of subsequent SARS-CoV-2 infections or vaccinations, was needed urgently. Furthermore, it would be beneficial to investigate the relationship between anti-COVID-19 antibody titers and immunity to infection or severe disease.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Influencing factors of Anti-SARS-CoV-2-Spike IgG antibody titres in healthcare workers – A cross-section study; Julia Reusch, Isabell Wagenhäuser, Alexander Gabel, Annika Eggestein, Anna Höhn, Thiên-Trí Lâm, Anna Frey, Alexandra Schubert-Unkmeir, Lars Dölken, Stefan Frantz, Oliver Kurzai, Ulrich Vogel, Manuel Krone, Nils Petri. medRxiv preprint 2022. DOI: https://doi.org/10.1101/2022.05.10.22274912, https://www.medrxiv.org/content/10.1101/2022.05.10.22274912v1
- Peer reviewed and published scientific report.
Reusch, Julia, Isabell Wagenhäuser, Alexander Gabel, Annika Eggestein, Anna Höhn, Thiên-Trí Lâm, Anna Frey, et al. 2022. “Influencing Factors of Anti-SARS-CoV-2-Spike-IgG Antibody Titers in Healthcare Workers: A Cross-Section Study.” Journal of Medical Virology 95 (1). https://doi.org/10.1002/jmv.28300. https://onlinelibrary.wiley.com/doi/10.1002/jmv.28300.