The SARS-CoV-2 infection causes a myriad of symptoms with varying levels of severity. Notably, CoV disease 2019 (COVID-19) produces a greater risk of severe illness in SOTRs because of their immunosuppressive therapies, and therefore SARS-CoV-2 vaccination is strongly advised in this group. Available data imply that the COVID-19 messenger ribonucleic acid (mRNA) vaccination is efficacious and safe for the general public. However, immunocompromised SOTRs have exhibited decreased immune responses following one or two SARS-CoV-2 vaccine doses.
Moreover, the third/booster dose of mRNA vaccine resulted in significant humoral responses and protected against catastrophic COVID-19 outcomes caused by the latest SARS-CoV-2 variants of concern (VOCs), like Omicron and Delta, in the general public. Nonetheless, the humoral responses induced by the booster dose in populations with weakened immune responses, notably SOTR, are less known.
About the study
In the present investigation, the researchers analyzed humoral responses in various SOTRs (liver, kidney, heart, and lung) after receiving the two and three doses of the SARS-CoV-2 mRNA vaccine in Canada. The team assessed humoral immune responses in groups of 11 liver, 31 kidney, eight heart, and 14 lung organ transplant recipients following the second (median 26 days) and third dose (median 35 days) COVID-19 mRNA vaccination.
According to the vaccination distribution standards of the public health authority of the province of Quebec, Canada, for immunocompromised individuals, the SOTRs got their initial two doses at varying intervals (median range 36 days) and their third dose roughly four months after the second shot. SOTR's humoral reactions to the vaccine were compared to those of a group of health care workers (HCWs) without COVID-19 history.
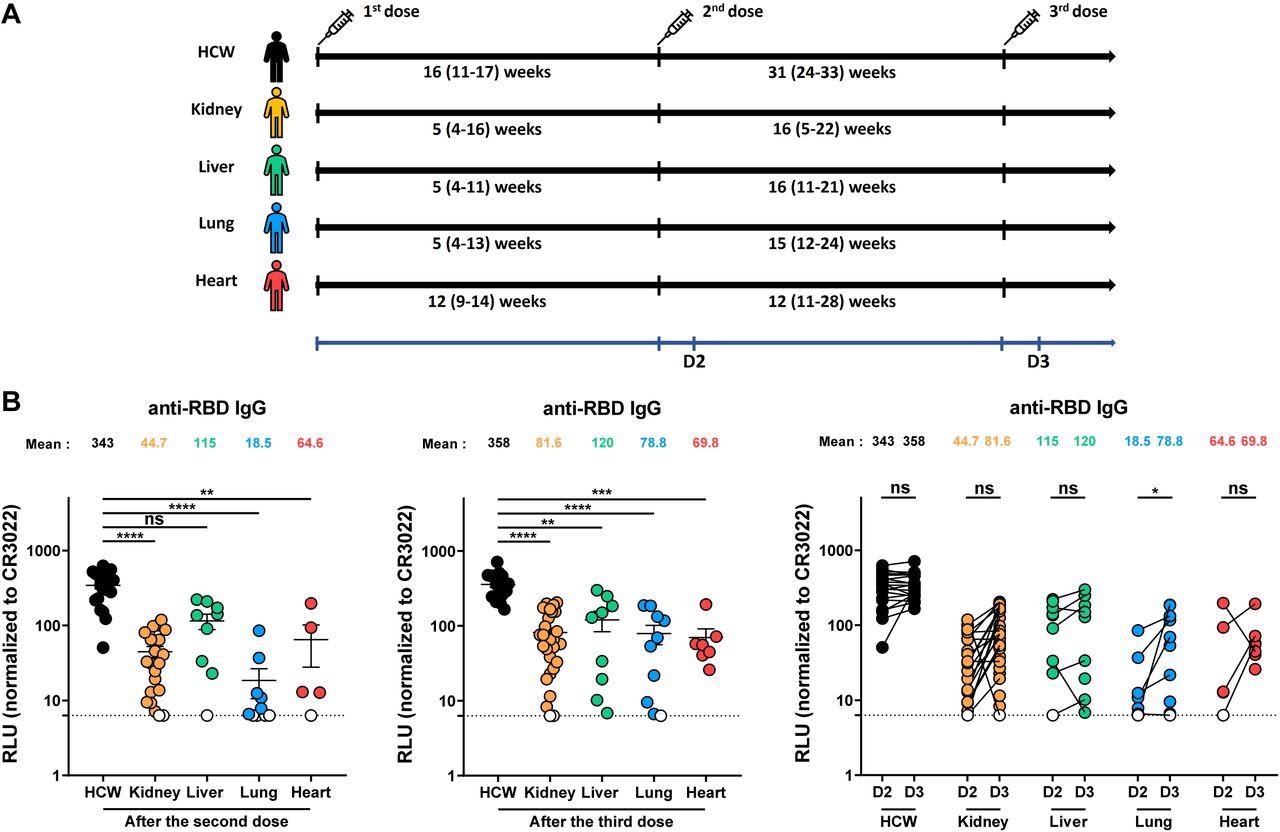
Elicitation of RBD-specific antibodies in SOTR and HCW after mRNA vaccination. (A) SARS-CoV-2 vaccine cohorts’ design. (B) Indirect ELISA was performed by incubating plasma samples from SOTR or HCW with recombinant SARS-CoV-2 RBD protein. Anti-RBD Ab binding was detected using HRP-conjugated anti-human IgG. Relative light unit (RLU) values obtained with BSA (negative control) were subtracted and further normalized to the signal obtained with the anti-RBD CR3022 mAb presents in each plate. Left panel shows the values obtained after the second dose, and middle panel after the third dose. Right panel shows the difference obtained between D2 (post-second dose) and D3 (post third dose) for every group. Symbols represent biologically independent samples from SOTR and HCW. Lines connect data from the same donor. Undetectable measures are represented as white symbols, and limits of detection are plotted. Error bars indicate means ± SEM. (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns, non-significant).
Based on the vaccination rollout recommendations for HCW in Quebec, the healthcare personnel received their initial two mRNA vaccine doses within a 16-week extended gap and their third dose about seven months following the second shot, demonstrating a long interval regimen for COVID-19 vaccination. This facilitated the authors to compare humoral responses observed in SOTR to those generated by a long interval vaccination schedule.
Vaccination-induced anti-SARS-CoV-2 receptor-binding domain (RBD)-targeting immunoglobulin Gs (IgGs) were quantified using a priorly established enzyme-linked immunosorbent assay (ELISA). Additionally, flow cytometry was used to assess the identification of the SARS-CoV-2 full-length spike (S) from the Omicron/Delta VOCs and D614G mutant following vaccination in HCW and SOTR. The team also assessed the S protein recognition of HKU1 βCoV, an endemic CoV that was significantly prevalent in the present population.
Moreover, the scientists determined the functional activities of the antibodies induced by vaccination and the anti-RBD avidity of these antibodies. Finally, they conducted an integrated assessment of vaccine responses observed in SOTRs.
Findings
Overall, the study results showed that relative to HCWs, SOTRs already known to react less effectively to SARS-CoV-2 vaccination because of their chronic immunosuppressive treatment displayed lower humoral responses following the second dose of the COVID-19 mRNA vaccine. Although the booster dose increased these reactions, they did not attain the same degree as HCWs.
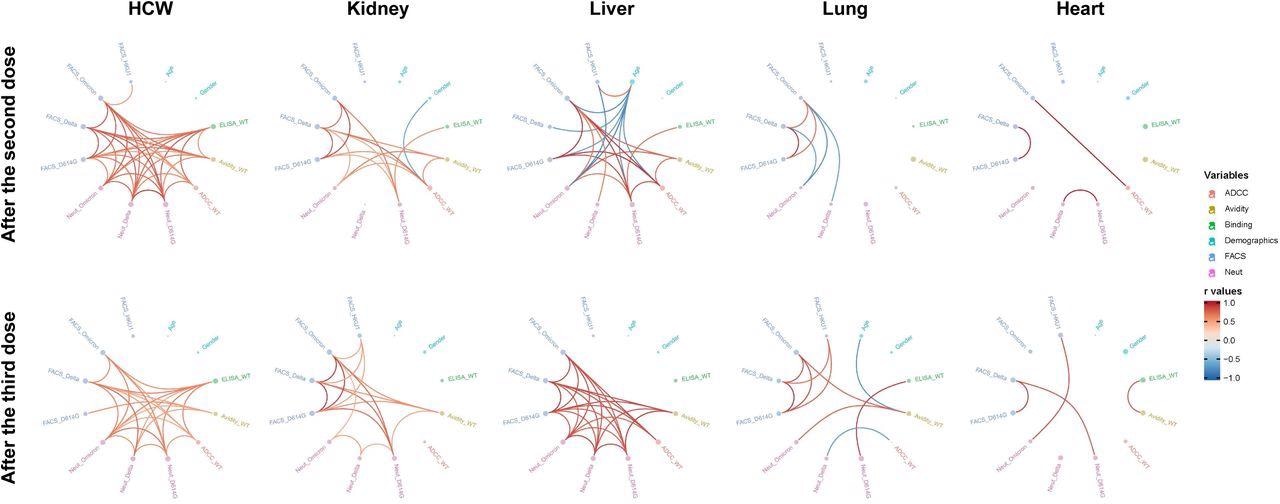
Mesh correlations of humoral response variables in SOTR and HCW after mRNA vaccination. Edge bundling correlation plots where red and blue edges represent positive and negative correlations between connected variables, respectively. Only significant correlations (p < 0.05, Spearman rank test) are displayed. Nodes are color-coded based on the grouping of variables according to the legend. Node size corresponds to the degree of relatedness of correlations. Edge bundling plots are shown for correlation analyses using ten different datasets, i.e., HCW and kidney, liver, lung or heart transplant recipients after the second and third doses of mRNA vaccination.
Following the third dose administration, the authors did not observe a significant hike in the range of neutralization and recognition of the SARS-CoV-2 VOCs in SOTRs, indicating an inability in antibody maturation. Further, low anti-RBD avidity was found in these patients, implying that their B cells were not as mature as those in HCW.
Interestingly, after the third shot of the mRNA vaccine, the team discovered that SOTR-generated antibodies with antibody-dependent cellular cytotoxicity (ADCC) activity were equivalent to HCWs. However, it was unclear if this was sufficient to safeguard SOTR from the detrimental consequences of COVID-19.
Depending on the transplanted organ, there were some variations in humoral responses. The humoral responses of liver recipients were much better than those of other SOTR cohorts. The lesser immunosuppressive therapies in liver recipients relative to the rest of the SOTR categories were most likely responsible for these disparities.
Conclusions
According to the study findings, expect liver recipients, the SARS-CoV-2 second dose vaccination generated poor humoral responses in SOTRs relative to a cohort of COVID-19 naïve immunocompetent HCWs. In addition, the authors found that the third vaccine dose increased humoral immunity in SOTRs, although not to the same extent in HCW.
While neutralizing efficacy against the SARS-CoV-2 Omicron and Delta variants remained low post-third vaccine shot, fragment crystallizable (Fc)-mediated effector activities in SOTR were comparable to those in the HCW cohort. Nevertheless, whether these responses will be enough to safeguard SOTRs from COVID-19-associated negative consequences was not clearly understood.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Humoral immune responses against SARS-CoV-2 Spike variants after mRNA vaccination in solid organ transplant recipients; Alexandra Tauzin, Guillaume Beaudoin-Bussieres, Shang Yu Gong, Debashree Chatterjee, Gabrielle Gendron-Lepage, Catherine Bourassa, Guillaume Goyette, Normand Racine, Zineb Khrifi, Julie Turgeon, Cecile Tremblay, Valerie Martel-Laferriere, Daniel E Kaufmann, Marc Cloutier, Renee Bazin, Ralf E. Duerr, Melanie Dieude, Marie-Josee Hebert, Andres Finzi. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.13.22275056, https://www.medrxiv.org/content/10.1101/2022.05.13.22275056v1
- Peer reviewed and published scientific report.
Tauzin, Alexandra, Guillaume Beaudoin-Bussières, Shang Yu Gong, Debashree Chatterjee, Gabrielle Gendron-Lepage, Catherine Bourassa, Guillaume Goyette, et al. 2022. “Humoral Immune Responses against SARS-CoV-2 Spike Variants after MRNA Vaccination in Solid Organ Transplant Recipients.” IScience 25 (9). https://doi.org/10.1016/j.isci.2022.104990. https://www.cell.com/iscience/fulltext/S2589-0042(22)01262-7.