Vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) essentially reduce morbidity and mortality and are crucial tools to contain the COVID-19 pandemic. FDA-approved vaccines are associated with a relatively small number of post-immunization adverse effects.
In the United States (US), reports to the Vaccine Adverse Event Reporting System (VAERS) consist of various systemic and neurologic manifestations. These adverse events are observed after mass vaccination programs and might have similar immunologic mechanisms with post-infection neurologic complications. Although rare, immune-mediated neurologic complications are less severe than after infection.
 Study: Neuropathic symptoms with SARS-CoV-2 vaccination. Image Credit: DOERS / Shutterstock
Study: Neuropathic symptoms with SARS-CoV-2 vaccination. Image Credit: DOERS / Shutterstock

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the current observational study, researchers clinically evaluated patients with new-onset paresthesia regardless of autonomic symptoms incident to COVID-19 vaccination. From January to September 2021, 23 patients were assessed for new onset of polyneuropathic symptoms within a month of SARS-CoV-2 vaccination. Medical records of the patients were abstracted to collect data. Excluded persons were those with recurrent neurologic symptoms or having non-neurologic complications and those at risk of developing dysautonomia and neuropathy.
Those with autonomic symptoms were subjected to standard autonomic nervous system (ANS) testing. Variability in heart rate after six to eight slow deep breaths per respiratory cycle was assessed. Tilt table tests were performed for 10 minutes following 20 minutes of supine rest. Postural orthostatic tachycardia syndrome (POTS) was defined as the sustained increase of 30 beats per minute or higher from the baseline after 10 minutes in the upright position without orthostatic hypotension. Two skin biopsies were removed from the lower leg to evaluate small fiber neuropathy (SFN).
Findings
The median age of the 23 subjects was 40 years, and a majority were women (21). None of the patients had prior neurologic illnesses. Five patients reported tachycardia, skin-flushing, and elevated blood pressure after administration of the COVID-19 vaccine, which lasted for 30 minutes or less and resolved entirely. All patients showed neurologic symptoms in at least 21 days following COVID-19 vaccination. Subjects were vaccinated with Pfizer’s BNT162b2, Moderna’s mRNA-1273, AstraZeneca’s ChAdOx1, or Janssen’s JNJ-78436735 vaccine. Fourteen patients showed neurologic symptoms after the first dose, while nine developed after receiving the second.
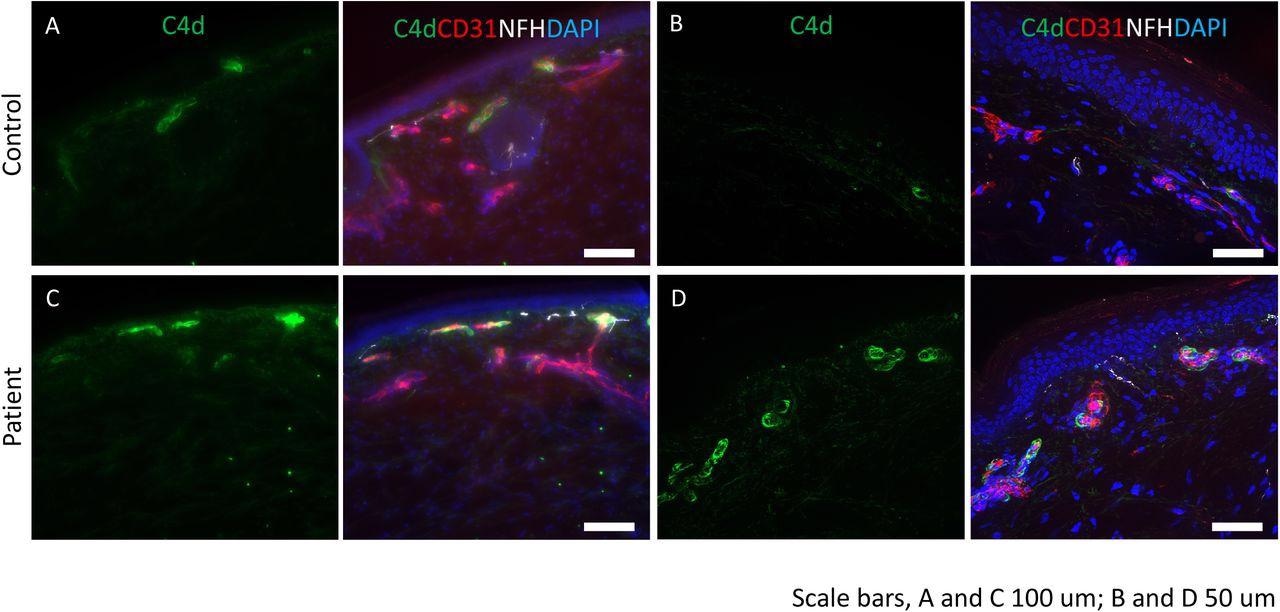
Complement deposition in skin of post-COVID-19 vaccine neuropathy: Immunostaining was performed for C4d (green), endothelial cell marker, CD31 (red) and neurofilament heavy chain, NFH (white). DAPI was used to stain the nuclei. (A and B) control tissues show minimal staining for C4d. CD31 identifies the endothelial cells in the blood vessels. (C and D) deposition of C4d is seen in the endothelial lining of the blood vessels. Scale bars: A and C are 100 mm and B and D are 50 mm.
All subjects complained of moderate to severe paresthesia and burning sensation in upper or lower limbs. About 60% of the participants developed autonomic symptoms, including new-onset Raynaud’s phenomenon, episodic tachycardia, and heat intolerance. Of the 12 patients undergoing ANS testing, abnormal findings were observed for 11 of them and six fulfilled the criteria for POTS. Seven subjects had diminished length-dependent sweat production, a characteristic feature of SFN. Magnetic resonance imaging (MRI) of the brain or spine available for 16 patients revealed no significant abnormalities.
Of the 16 participants who underwent skin biopsies, five patients showed subthreshold nerve fiber density, two with borderline density at the distal side of the leg, and three showed axonal swellings in the fibers—all of these exhibited nerve conduction velocities which confirmed small-fiber axonal neuropathy. Moreover, C4d complement deposition on endothelial cells was observed in five patients compared to nine age-matched controls. Twelve patients were treated with oral corticosteroids; seven received a standard prednisone dose for a week with a subsequent taper of 20% of the initial dose showed significant improvement in neurologic symptoms after two weeks.
Three patients who showed persistent symptoms of dysautonomia and SFN for five to nine months were managed on intravenous immunoglobulin (IVIg) treatment. IVIg treatment was significant as symptoms improved in two weeks, resolving entirely for one of them and remaining as mildly residual in the other two. Among the non-recipients of immune therapies, partial recovery was evident in seven patients, complete in just one participant (by 12 weeks post-onset of symptoms), and three had no improvement.
Conclusions
The authors observed that all patients experienced neuropathic symptoms within three weeks of vaccination. However, referral bias limits the findings given the study’s observational nature, and the lack of a control group precludes attributing a causative role despite the temporal association of vaccines to symptoms.
Notably, oligoclonal bands in two of the five tested patients’ cerebrospinal fluid, deposition of immune complexes (C4d), and response to immunotherapy suggest a possible immune association. Although some patients responded positively to corticosteroids or IVIg treatment, their use should be cautiously monitored or viewed in the context of clinical trials. Further research is necessary, however, to determine whether the SARS-CoV-2 vaccine causes neuropathies.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Neuropathic symptoms with SARS-CoV-2 vaccination, Farinaz Safavi, Lindsey Gustafson, Brian Walitt, Tanya Lehky, Sara Dehbashi, Amanda Wiebold, Yair Mina, Susan Shin, Baohan Pan, Michael Polydefkis, Anne Louise Oaklander, Avindra Nath. medRxiv 2022, DOI: https://doi.org/10.1101/2022.05.16.22274439, https://www.medrxiv.org/content/10.1101/2022.05.16.22274439v1