Background
The COVID-19 pandemic has transformed research objectives in a variety of scientific fields, including maternal-infant health. For example, key research priorities have shifted to the generation of efficient vaccines to halt the transmission of SARS-CoV-2, understand transmission pathways, determine the efficacy of infection prevention techniques, and discover treatments to alleviate symptoms.
In this regard, lactating women who have previously been infected with SARS-CoV-2 or have been vaccinated against COVID-19 have been of interest to researchers.
Previous studies have confirmed the absence of SARS-CoV-2 genetic material in breast milk, thus indicating that breastfeeding is safe for the infant. However, specific SARS-CoV-2 antibodies have been found in breast milk, which suggests that lactation could be a route for antibody transfer that protects the breastfeeding infant.
While IgA antibodies make up most antibodies found in human milk, researchers have been increasingly interested in the role of other immunoglobulins like IgG and its isotypes in breast milk.
About the study
Breast milk samples were obtained from ten breastfeeding women who had previously been vaccinated against COVID-19. Within the vaccination priority groups set by the Spanish government, immunized participants received two doses of either the Pfizer or Moderna messenger ribonucleic acid (mRNA) COVID-19 vaccines.
Pre-pandemic breast milk samples were also obtained from ten nursing mothers. These samples were also used to define cut-off values for anti-SARS-CoV-2 IgA and IgG antibodies. Prior SARS-CoV-2 infection in these samples was verified by reverse-transcription-polymerase chain reaction (PCR) assay.
Static in vitro digestion was performed on whole breast milk samples. The gastrointestinal stage was recreated in the milk sample by adding simulated gastric fluid containing pepsin and gastric lipase. All samples were then placed in a thermostatic compartment at 37 °C with agitation at 55 runs per minute (rpm) for 60 minutes during the gastric stage.
The intestinal stage was then initiated by adding simulated intestinal fluid that consisted of pancreatin and bile salts in a 4:6 ratio. After correcting the pH to 6.6 with sodium hydroxide (NaOH), the samples were then placed in the agitation chamber for another 60 minutes to resemble the intestinal stage.
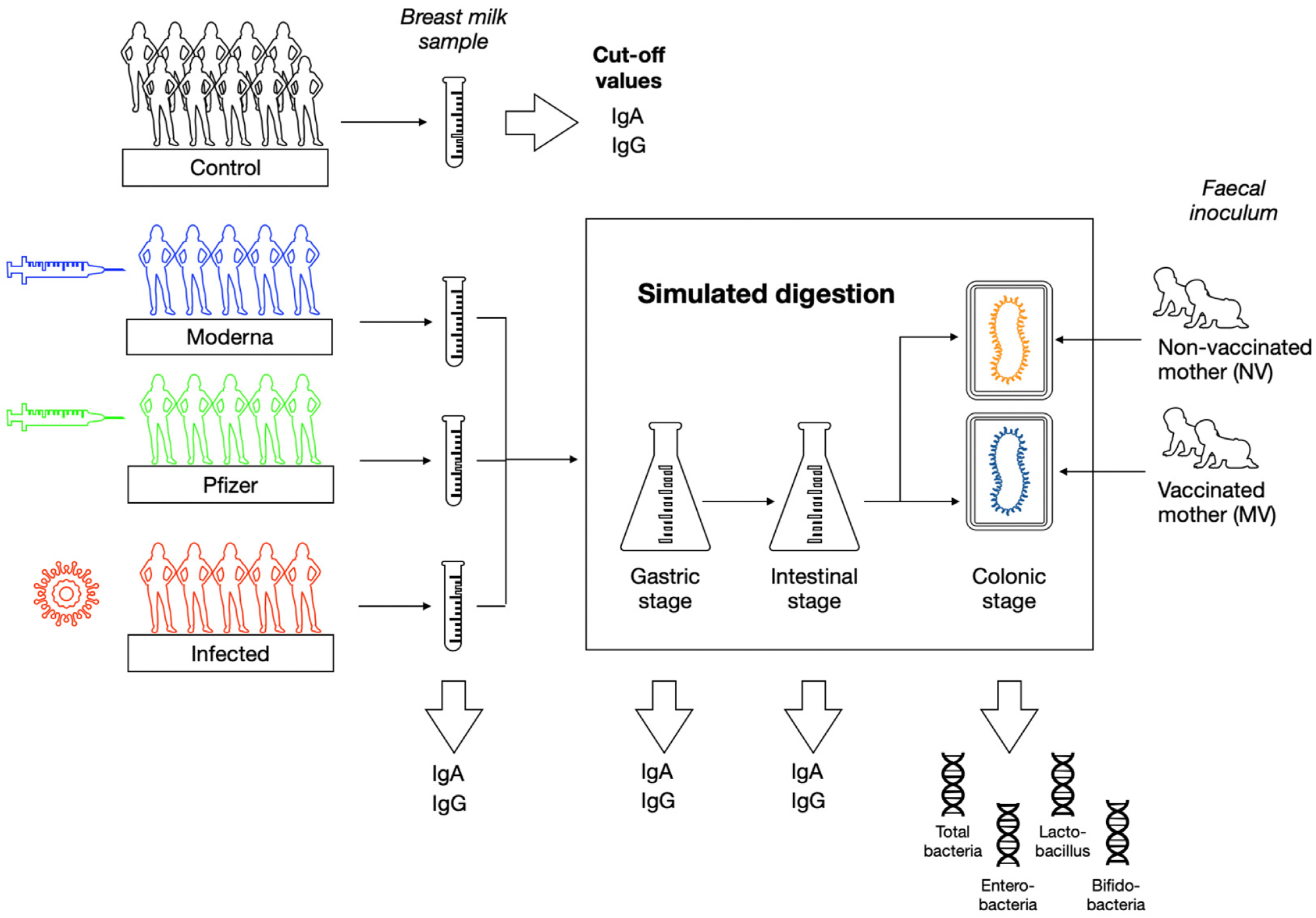 Overview of the study design.
Overview of the study design.
Study findings
Anti-SARS-CoV-2 IgA levels in breast milk samples before simulated digestion were 132 and 121 arbitrary units (AU), with no statistically significant differences between the Pfizer and Moderna vaccinated groups, respectively. Comparatively, a median 401 AU IgA was observed in the infected group.
Comparatively, basal IgG levels in the infected group were significantly lower at a median of 188 AU as compared to median IgG levels of 511 AU and 740.9 AU in breast milk samples obtained from mothers who received the Pfizer and Moderna vaccines, respectively.
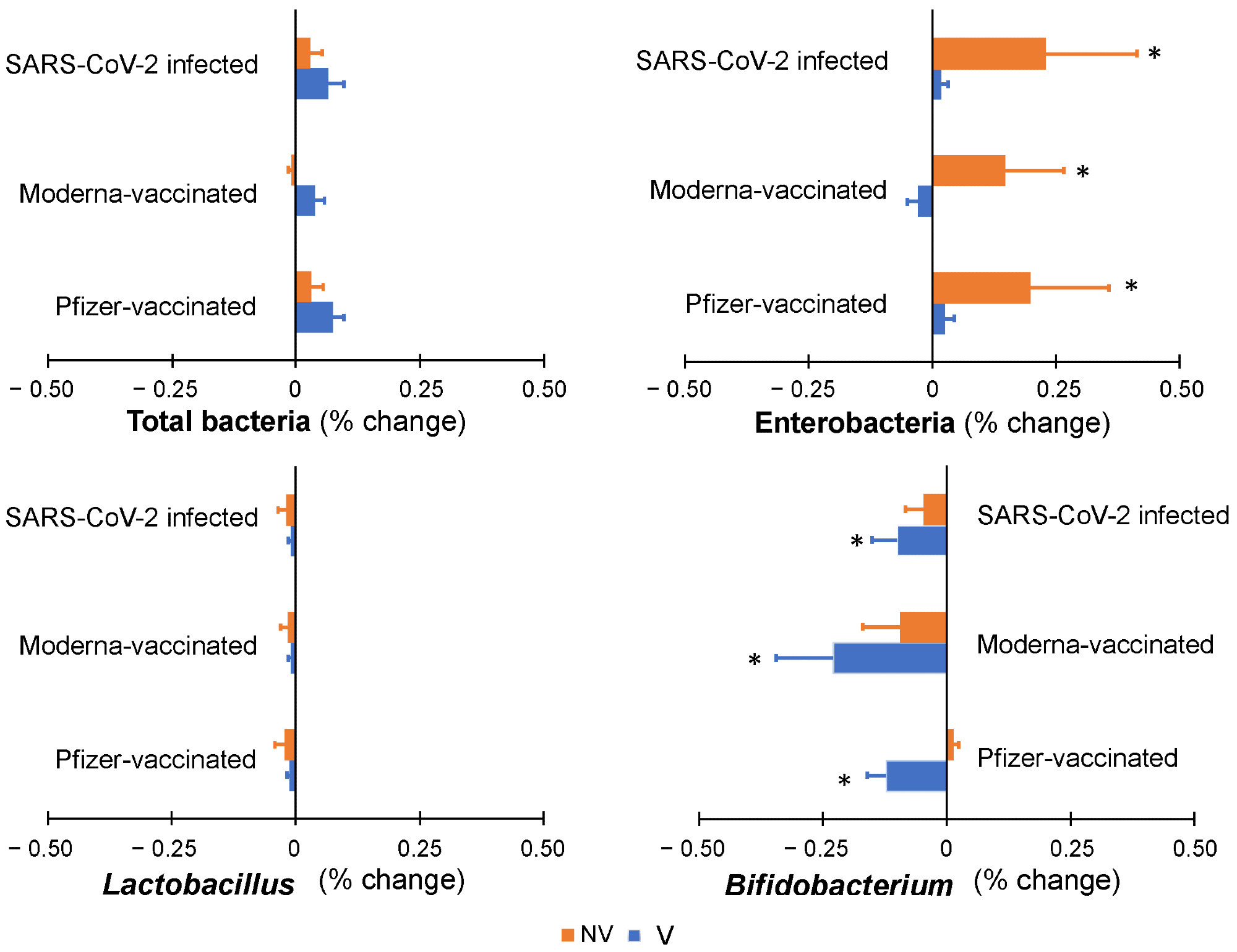 Colonic microbiota profile along simulated colonic fermentation of infants from vaccinated (V) and non-vaccinated or infected (NV) mothers, using as substrates digested breast milk samples in previous gastrointestinal simulation, containing anti-SARS-CoV-2 antibodies as a result of maternal vaccination (with Pfizer or Moderna) or infection. * indicates statistically significant differences with respect to baseline values prior to colonic fermentation.
Colonic microbiota profile along simulated colonic fermentation of infants from vaccinated (V) and non-vaccinated or infected (NV) mothers, using as substrates digested breast milk samples in previous gastrointestinal simulation, containing anti-SARS-CoV-2 antibodies as a result of maternal vaccination (with Pfizer or Moderna) or infection. * indicates statistically significant differences with respect to baseline values prior to colonic fermentation.
After the in vitro intestinal stage digestion, the levels of both anti-SARS-CoV-2 IgA and IgG significantly declined. Although the IgA levels were initially higher in breast milk samples from infected mothers, these levels declined to levels that were comparable to the samples obtained from vaccinated mothers. More specifically, following intestinal digestion, IgA values were 41 AU in the infected group, and 36 AU and 61 AU in Moderna and Pfizer vaccine recipients, respectively.
The final levels of anti-SARS-CoV-2 IgG after simulated digestion were significantly decreased. However, these levels maintained the same proportion among both groups as the previous value in the breast milk samples before digestion.
Moreover, anti-SARS-CoV-2 IgG levels in samples from women who received the Moderna and Pfizer COVID-19 vaccines were 70 and 54 AU, respectively, as compared to 12 AU in samples from infected mothers.
Implications
The study findings reveal that, despite lower levels, anti-SARS-CoV-2 antibodies in breast milk persist during gastrointestinal digestion. Despite the in vitro setting and small sample size, the current study suggests that anti-SARS-CoV-2 antibodies in breast milk may have a protective effect in babies born to women vaccinated against COVID-19, as well as previously infected women.
Journal reference:
- Calvo-Lerma, J., Bueno-Llamoga, P., Bäuerl, C., et al. (2022). Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients. doi:10.3390/nu14102117