COVID-19 vaccines have been effective in decreasing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, transmission, hospitalizations, and deaths. The likelihood of long COVID may be lower among individuals who are infected by SARS-CoV-2 after vaccination; however, the association between COVID-19 vaccination and long COVID symptoms is not clear.
 Study: Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. Image Credit: Donkeyworx / Shutterstock
Study: Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. Image Credit: Donkeyworx / Shutterstock
About the study
In the present community-based observational cohort study, researchers assessed the likelihood of experiencing long COVID symptoms and the impact of long COVID on the performance of daily activities among UK community residents with SARS-CoV-2 infections before COVID-19 vaccination.
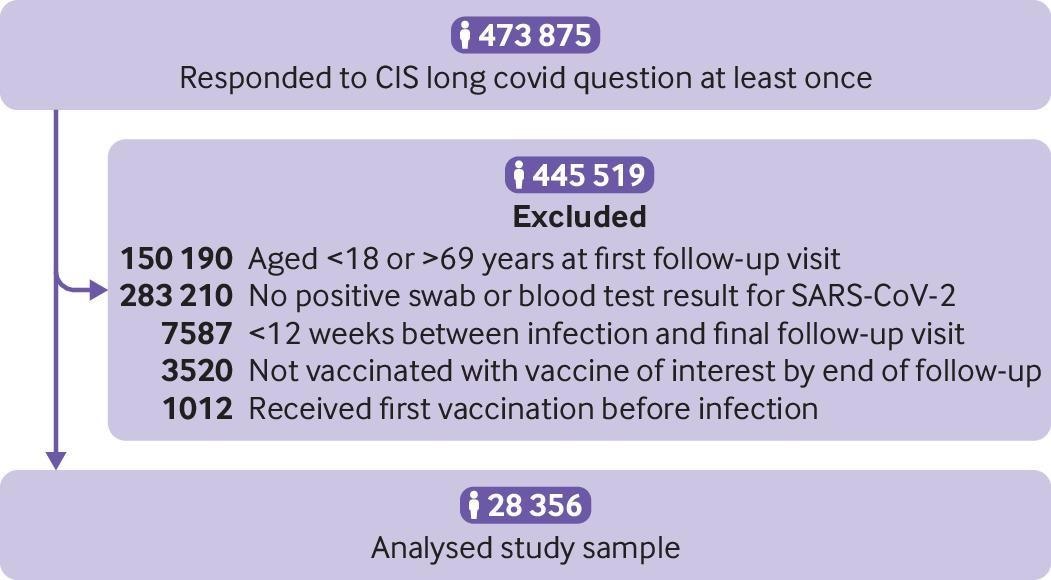
Study participant flow diagram. CIS=Office for National Statistics COVID-19 Infection Survey
The study comprised 18-to-69-year-old individuals who had participated in the COVID-19 Infection Survey, which involved UK households (excluding communal establishments such as care homes. hospitals, care homes, prisons, and residence halls). Vaccination data (vaccine doses, vaccination date, and vaccine manufacturers) were obtained from the COVID-19 Infection Survey and the National Immunisation Management System for participants residing in England.
At each monthly follow-up visit, the participants were asked if they experienced any symptoms of long COVID (described as symptoms persisting for a minimum of four weeks after suspected or confirmed COVID-19 which could not be explained by any other health condition). The survey respondents also provided self-collected nasopharyngeal and oropharyngeal swab samples for reverse transcription-polymerase chain reaction (RT-PCR) testing at each follow-up visit.
The primary outcome measure was the presence of long COVID symptoms for a minimum of 12 weeks after SARS-CoV-2 infection and during follow-up between February 3 and September 5, 2021. The secondary outcome measure was limitations in performing daily activities due to long COVID. In addition, the team evaluated 10 symptoms that were most frequently reported during follow-ups and if the participants experienced >3 or >5 of the 21 long COVID symptoms included in the survey.
All the participants were vaccinated with either a single dose of an adenovirus vector COVID-19 vaccine (ChAdOx1 nCoV-19) or messenger ribonucleic acid (mRNA) vaccine (BNT162b2 or mRNA-1273) after testing SARS-CoV-2-positive.
The survey questions asked about symptoms of long COVID which persisted for >4 weeks after SARS-CoV-2 infection; however, for the analysis, a 12-week period was used, in accordance with the World Health Organization (WHO) definition of the post-COVID-19 condition and the UK clinical case definition of the post-COVID-19 syndrome.
Results
Among the 28,356 study participants, the average age was 46 years and 56% (n=15,760) of them were females. The majority of the participants (89%) were Whites. The average follow-up periods were 141 days and 67 days after the first and second COVID-19 vaccinations, respectively.
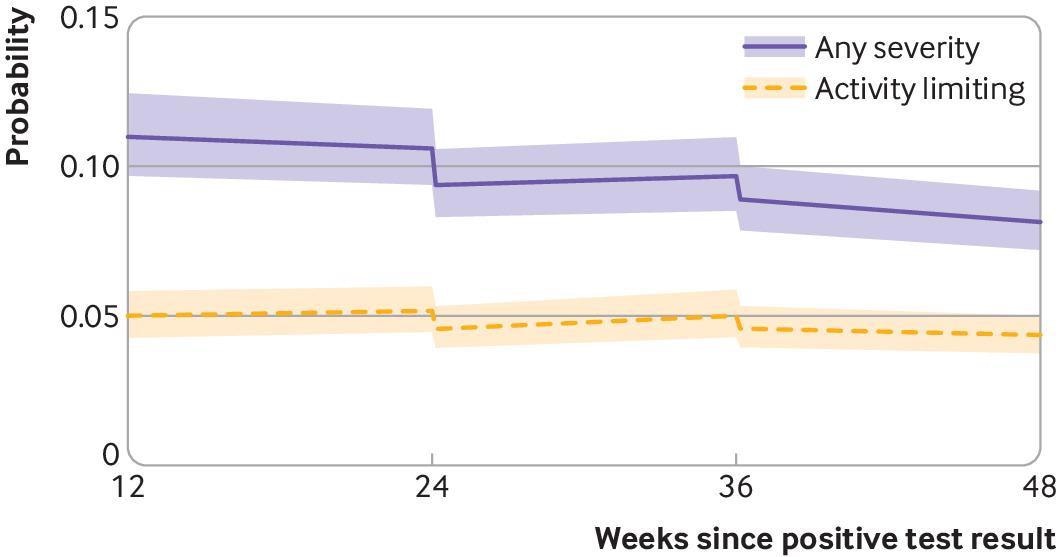
Modeled probabilities of long covid for a hypothetical study participant who received a first covid-19 vaccine dose 24 weeks after SARS-CoV-2 infection and a second dose 12 weeks later. Probabilities are shown for participants of mean age (50 years) and in the modal group for other covariates (woman, white ethnicity, resident in London, resident in an area in the least deprived fifth group, not a patient-facing health or social care worker, no pre-existing health conditions, not admitted to hospital during the acute phase of infection, infected on 7 September 2020). Although estimated probabilities are specific to this profile, proportional changes in probabilities after vaccination do not vary across characteristics and can therefore be generalized to other profiles. Dashed lines represent timing of vaccination. Shaded areas are 95% confidence intervals
A total of 6,729 participants (24%) experienced symptoms of long COVID a minimum of once during the follow-up period. After the first vaccination, a 13% reduction was observed in the likelihood of long COVID symptoms, followed by elevations and reductions in the trajectory of long COVID symptoms (ranging between 0.3% and 1.2% weekly). After the second vaccination, a 9% initial reduction was observed in the likelihood of long COVID symptoms, followed by further reductions of 0.8% weekly.
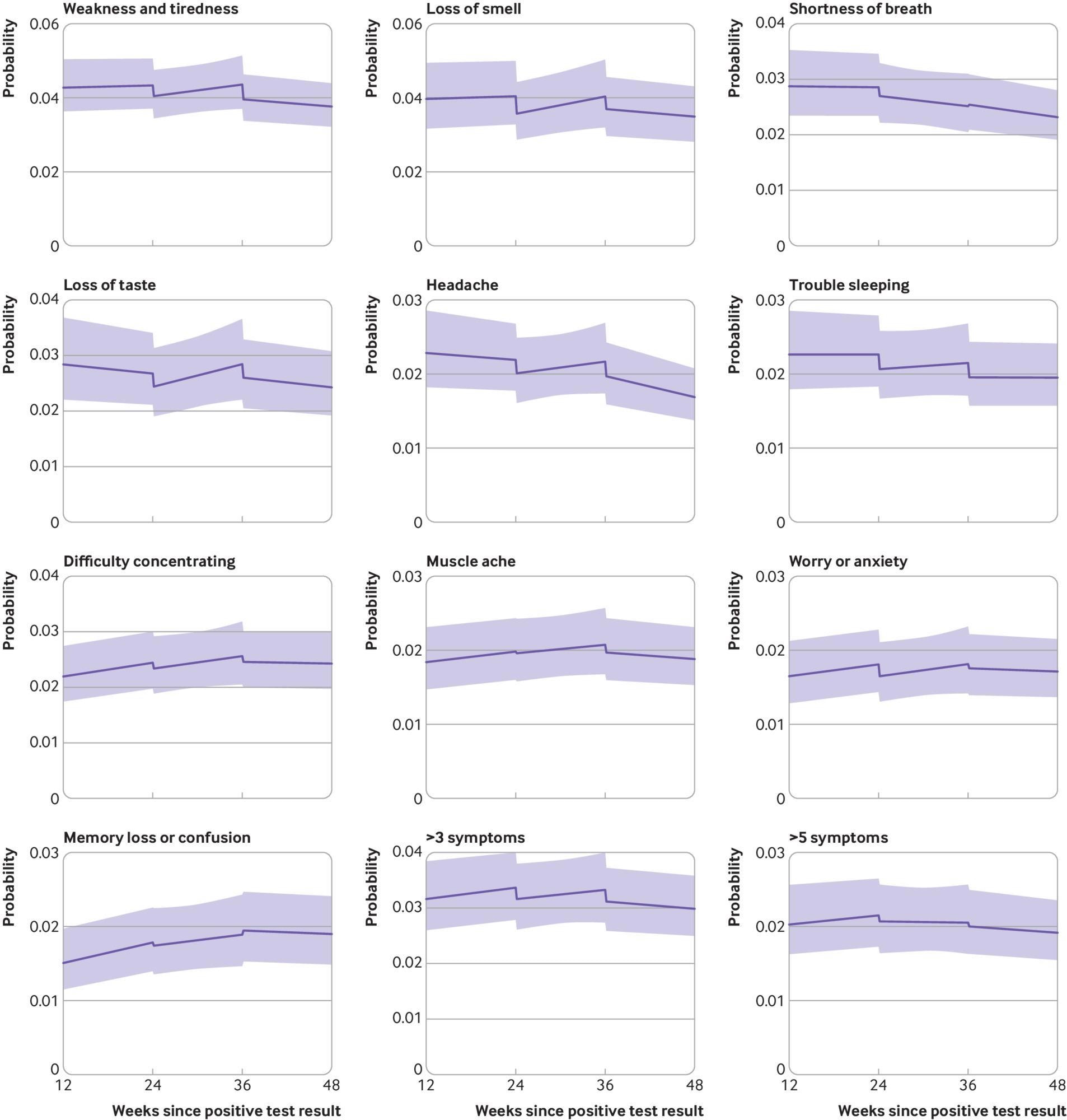 Modeled probabilities of individual long covid symptoms for a hypothetical study participant who received the first dose of a covid-19 vaccine 24 weeks after SARS-CoV-2 infection and a second dose 12 weeks later. Top 10 most frequently reported symptoms ordered by modeled probability at 12 weeks post-infection. Probabilities are shown for a participant of mean age (50 years) and in the modal group for other covariates (woman, white ethnicity, resident in London, resident in an area in the least deprived fifth group, not a patient-facing health or social care worker, no pre-existing health conditions, not admitted to hospital during the acute phase of infection, infected on 7 September 2020). Although the estimated probabilities are specific to this profile, proportional changes in probabilities after vaccination do not vary across characteristics and can therefore be generalized to other profiles. Dashed lines represent the timing of vaccination. Shaded areas are 95% confidence intervals
Modeled probabilities of individual long covid symptoms for a hypothetical study participant who received the first dose of a covid-19 vaccine 24 weeks after SARS-CoV-2 infection and a second dose 12 weeks later. Top 10 most frequently reported symptoms ordered by modeled probability at 12 weeks post-infection. Probabilities are shown for a participant of mean age (50 years) and in the modal group for other covariates (woman, white ethnicity, resident in London, resident in an area in the least deprived fifth group, not a patient-facing health or social care worker, no pre-existing health conditions, not admitted to hospital during the acute phase of infection, infected on 7 September 2020). Although the estimated probabilities are specific to this profile, proportional changes in probabilities after vaccination do not vary across characteristics and can therefore be generalized to other profiles. Dashed lines represent the timing of vaccination. Shaded areas are 95% confidence intervals
Long COVID symptoms resulting in limitation of daily activities were reported by 4,747 participants (17%) a minimum of once during the follow-up period. The first vaccination was associated with an initial 12% decrease in the likelihood of daily activity limitation which was followed by an uncertain trajectory (0.9%weekly) till the second vaccination. The second vaccination was associated with an initial 9% decrease in the likelihood of daily activity limitation, followed by -0.5% weekly till termination of the follow-up period.
No statistically significant differences were found in the association between COVID-19 vaccinations and long COVID symptoms by health-related factors, sociodemographic characteristics, hospitalization with acute SARS-CoV-2 infections, vaccination type (mRNA or adenovirus vector vaccine), or duration between COVID-19 and its vaccination.
The odds of experiencing >3 or >5 symptoms of long COVID initially decreased after the first and the second vaccination. After the first vaccination, the largest decreases were noted for anosmia (−13%), ageusia (−9%), and poor sleep (−9%). After the second vaccination, the most significant decreases were noted for fatigue (−10%), headaches (−9%), and poor sleep (−9%).
Overall, the study findings showed that the odds of long COVID symptoms decreased after SARS-CoV-2 vaccination, with sustained immunity after the second vaccination, at least during the mean 67-day- follow-up period. The results underpin the importance of vaccination to reduce the long COVID healthcare burden. However, further research with more extended periods of follow-up is required.
Journal reference:
- Daniel Ayoubkhani, Charlotte Bermingham, Koen B Pouwels, Myer Glickman, Vahé Nafilyan, Francesco Zaccardi, Kamlesh Khunti, Nisreen A Alwan, A Sarah Walker. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study, BMJ2022;377:e069676. DOI: 10.1136/bmj-2021-069676, https://www.bmj.com/content/377/bmj-2021-069676