The term 'NeuroCOVID' encompasses the neuropathologic, cognitive, and neuropsychiatric symptoms of coronavirus disease 2019 (COVID-19) and Long COVID. Post-mortem studies have shown deceased COVID-19 patients' brains with cerebrovascular abnormalities, including blood-brain barrier (BBB) permeability, T-cell infiltration, gliosis, neurons, and synapse losses.
Likewise, studies have shown that middle and advanced-age individuals are highly susceptible to NeuroCOVID. Accordingly, COVID-19 patients between 36 to 65 years have elevated endothelial inflammation and neuronal injury markers in their serum two months after contracting mild COVID-19. Similarly, advanced age COVID-19 patients also suffer from anxiety, post-traumatic stress disorders, and dementia.
BBB, which selectively restricts the permeability of central nervous system (CNS) blood vessels to macromolecules and immune cells from the blood, gets damaged from the loss of endothelial-tight junctions. Thus, age-related decline in cerebrovascular function and BBB integrity increases susceptibility to NeuroCOVID.
About the study
In the present study, researchers evaluated the effect of age and infectious SARS-CoV-2 on the BBB in vivo, hypothesizing that age-related declines in BBB function and wingless-related integration site (Wnt)/β-catenin exacerbate NeuroCOVID. Note that Wnt/β-catenin promotes adult BBB maintenance and repair, and SARS-CoV-2 infection can compromise the BBB.
The team intranasally inoculated two-month (young) and 12-month-old (middle-age) C57Bl/6 mice. They used a mouse-adapted SARS-CoV-2 strain for their experiments to cause pulmonary infection in the test animals.
The researchers measured neurologic and neuropathologic outcomes in mice at the peak of viral replication in the lung, i.e., four days post-inoculation (4DPI). After 4DPI, they euthanized mice and prepared sagittal brain sections for histology. They also conducted immunostaining for cerebrovascular basement membrane Collagen IV with nuclear counterstain 4′,6-diamidino-2-phenylindole (DAPI).
The researchers performed a reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for SARS-CoV-2 viral ribonucleic acid (RNA) at 4 DPI. In addition, the team measured foci of inflammation in the BBB, blood-meningeal barrier, and blood-cerebral spinal fluid barrier and assessed the neuroanatomic distribution of T cells in the CNS.
Study findings
The authors observed SARS-CoV-2 RNA in multiple CNS tissues but could not detect viral RNA in the lungs. They also found that pathogen-associated molecular patterns were highly expressed in the brain after SARS-CoV-2 infection and did not differ by age.
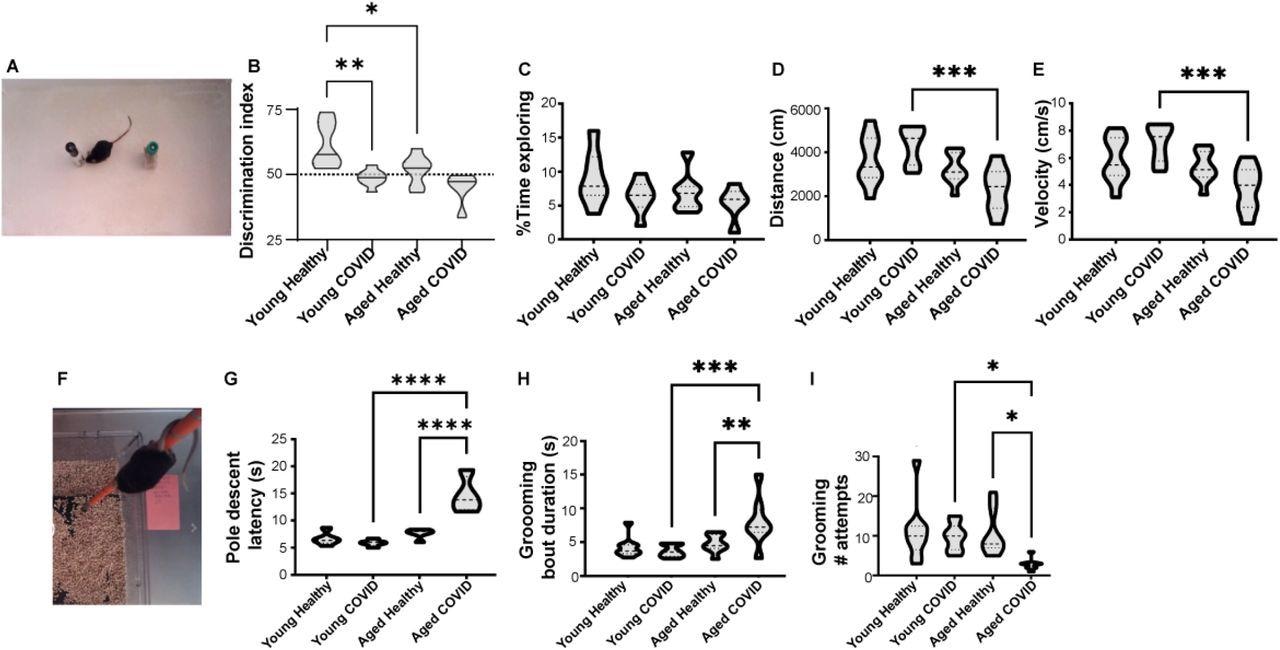
SARS-COV-2 respiratory infection causes neuropsychiatric abnormality in middle-aged but not young adult mice. A) Mouse with novel and familiar objects in open field behavior arena. B) Novel object recognition memory task. Young healthy mice preferentially attend to novel object (Discrimination Index >50% indicated by dotted horizontal line). Young mice infected with SARS-CoV-2, middle-aged healthy mice, and middle-aged mice infected with SARS-CoV-2 do not prefer the novel object. (n=5-8 per group) C) No significant differences in total exploration time between groups (n=5-8 per group). D) Decreased cumulative distance traveled by voluntary ambulation in the 12-month-old SARS-COV-2 mice (n=8-9 per group). E) Velocity of voluntary movement in the open field (n=8-9 per group). F-G) 12-month-old infected mice have significantly increased latency in the pole descent task, a complex motor coordination task involving brainstem/thalamic connectivity (n=5 per group). H) Duration of each bout of spontaneous grooming in the open field (n=8-9 per group). I) Number of spontaneous grooming bouts initiated in the open field (n=8-9 per group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, one-way ANOVA and Sidak’s multiple comparisons test.
A robust canonical Wnt activity is needed to maintain the resilience of young adults to SARS-CoV-2 infection. The BBB permeability in aged infected mice was due to loss of Wnt3 and Wnt7a suppression of Caveolin-1, which could exacerbate inflammation by enhancing the delivery of viral material across the BBB. The study data suggested that loss of Wnt-β-catenin in the aging brain created vulnerability to NeuroCOVID.
Caveolin-1 is a scaffolding protein that promotes transcellular BBB permeability whereas brainstem Zonula occludens-1 (ZO-1) is a protein that stably links junctional proteins to the cytoskeleton to restrict macromolecular permeability. SARS-CoV-2 infection in middle-aged mice induced the BBB transcytosis protein Caveolin-1 and downregulated ZO-1. However, the infection caused no apparent changes to tight junction proteins. Overall, this data indicated that age exacerbated transcellular BBB leakage caused by SARS-CoV-2 infection.
Further, SARS-CoV-2 infection induced a two- to three-fold increase in parenchymal foci of hypercellularity. Compared to young mice, 12-month-old infected mice had 35 perivascular inflammatory foci per brain section and ~30% more parenchymal inflammatory foci. The authors also found a few inflammatory foci in the choroid plexus, which indicated age exacerbated SARS-CoV-2-induced parenchymal inflammation.
Furthermore, the team observed a four-fold increase in a cluster of differentiation 3 (CD3)+ T cell infiltration of the brainstem and the olfactory bulb in SARS-CoV-2 infected 12-month-old mice compared to younger infected mice.
Conclusion
According to the authors, this is the first study to experimentally test the impact of age on SARS-CoV-2-induced neuropathology in a small animal model. The authors evidenced that age worsened dysregulated Wnt/β-catenin signaling and BBB permeability.
Intriguingly, brain endothelial cell transcriptomic signatures are conserved between COVID-19 and Alzheimer's disease. Moreover, cerebrovascular damage caused by SARS-CoV-2 infection could accelerate vascular cognitive impairment and dementia (VCID). It is particularly worrisome that 1.6% of adults over 65 years were diagnosed with new-onset dementia in the first three months after SARS-CoV-2 infection. Moreover, it was 2.4 times more common after COVID-19 than other acute health events. In the coming years, the additive effect of increased neuropsychiatric diseases, including COVID-related dementia, could be staggering. To conclude, age aggravated the BBB disruption, neuroinflammation, and neuropsychiatric presentation in SARS-CoV-2 infected mice. These findings necessitate the identification of potential new therapeutic strategies to increase the resiliency of the aging brain.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Age exacerbates SARS-CoV-2-induced blood-brain barrier leakage and neuropsychiatric dysfunction, Seshadri Bhargava Niladhuri, Guliz Otkiran Clare, KaReisha F. Robinson, Jacob Class, Ali A Almousawi, Troy N Trevino, Felecia Marottoli, Leon M. Tai, Justin ME Richner, Sarah E Lutz, bioRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.06.02.494552 https://www.biorxiv.org/content/10.1101/2022.06.02.494552v1