The SARS-CoV-2-induced coronavirus disease 2019 (COVID-19) pandemic prompted the invention of numerous vaccinations that safeguard billions of humans from the disease's severe course, primarily through the generation of humoral or antibody-triggered immunity. T-cell immunity is critical for controlling SARS-CoV-2 infection, especially in individuals who cannot generate a humoral immune response to a prophylactic vaccination or natural infection. Individuals with inherited B-cell deficiency and cancer patients with treatment- or disease-linked B cell reduction fall into this category. T cells are crucial for COVID-19 outcomes and SARS-CoV-2 immunity maintenance in addition to B cell-driven humoral immunity.
CoVac-1, a peptide-based T-cell activator comprising of SARS-CoV-2 T-cell epitopes obtained from several viral proteins, coupled with the toll-like receptor 1 and 2 (TLR 1/2) agonist XS151, had a favorable efficacy and safety profile in a phase 1 trial among healthy adults considering the induction of COVID-19-specific T-cell responses, according to the authors of the current study. This T cell response was superior to those induced by COVID-19 or currently approved SARS-CoV-2 vaccines.
CoVac-1 comprises various SARS-CoV-2 human leukocyte antigen (HLA)-DR T cell epitopes generated from distinct SARS-CoV-2 proteins, such as nucleocapsid (N), spike (S), membrane (M), envelope (E), open reading frame 8 (ORF 8). Hence, CoVac-1 stimulates T-cell immunity autonomous of current SARS-CoV-2 variants of concern (VOCs).
About the study
In the current study, the researchers ran a Phase 1/2 open-label CoVac-1 experiment enrolling 54 individuals with acquired or congenital B-cell deficiency who received a single subcutaneous CoVac-1 dose in Germany. The study's primary aim was immunogenicity in terms of CoVac-1-triggered T-cell responses till day 28; the secondary objective was safety till day 56. In addition to B-cell deficiency, half of the patients had CD4 + T cell counts of less than 500/µl, highlighting the trial population's severe immunodeficiency.
The team evaluated the data on adverse events from the patients. T-cell reactions toward the six SARS-CoV-2 HLA-DR CoVac-1 T cell epitopes were measured utilizing enzyme-linked immunosorbent spot (ELISPOT) evaluations to determine immunogenicity. T-cell responses were measured in all eligible subjects at baseline/day 1, day 7, 14, and 28 following receiving CoVac-1.
Results
The study results showed that 94 patients with acquired or congenital B-cell deficiency were screened at three research sites in Germany from 6 July 2021 to 13 January 2022. In addition, CoVac-1 was given to 54 patients, 14 in the Phase 1 safety run-in and 40 in the Phase 2 portion of the experiment.
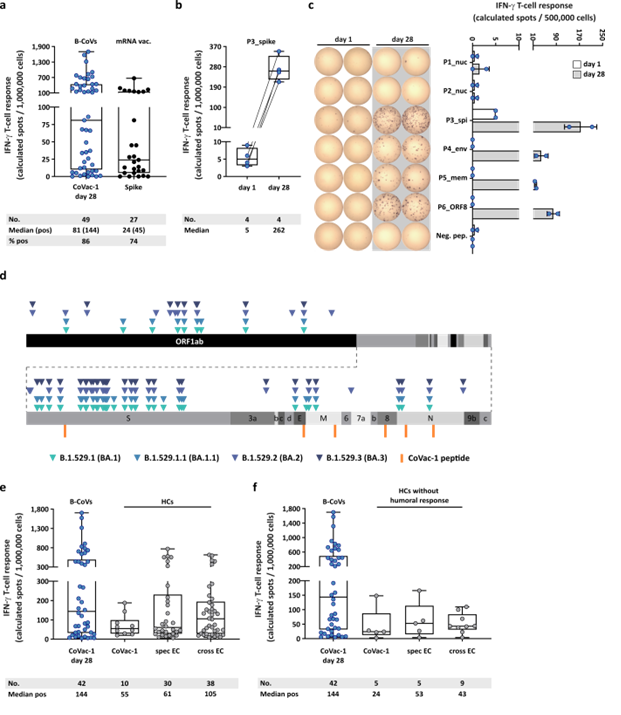
CoVac-1-induced T-cell responses with regard to Omicron variants and compared to mRNA vaccine- or infection-induced T-cell response. (a) CoVac-1-specific T-cell responses assessed ex vivo in study patients (day 28) compared to spike-specific T-cell responses prior to CoVac-1 administration in patients after second or third vaccination with approved mRNA vaccines (n = as indicated). (b) Intensities of P3_spike-induced IFN‑g T-cell responses assessed ex vivo in study patients (n = as indicated) prior to and on day 28 after CoVac-1 administration. (c) Exemplary ex vivo ELISPOT assays of one study patient (UPN12), with pre-existing T-cell responses to P3_spike, for the six CoVac-1 peptides on day 1 (white) and day 28 (grey). The intensities of IFN‑g T-cell responses are depicted as calculated spot counts (mean spot count of technical replicates minus the respective negative control). (d) Color-coded mutations described for SARS-CoV-2 Omicron variants are shown together with CoVac-1 peptides (orange). Positive T-cell responses to specific (spec) and cross-reactive (cross) T-cell epitope compositions (ECs) in (e) immunocompetent HCs (CoVac-1, spec EC, cross EC, n = as indicated)10,11 and (f) immunocompetent HCs without anti-SARS-CoV-2-antibody response after infection (CoVac-1, spec EC cross EC, n = as indicated) compared to positive IFN-g T-cell responses in study patients assessed ex vivo (B-CoVs, n = as indicated, day 28). (a,b,e,f) The intensity of IFN‑g T-cell responses is depicted as calculated spot counts (mean spot count of technical replicates minus the respective negative control). Box plots or combined box-line plots show median with 25th or 75th percentiles, and min/max whiskers. (c) Bars with mean, SD and single data points. no., number; EC, epitope composition; HCs, healthy COVID-19 convalescents; ORF, open reading frame; pos, positive.
Details on unsolicited and solicited adverse events were available for every participant via diary cards for 28 days following CoVac-1 administration and safety checkups till day 56. No subject dropped out of the study due to side effects. The authors did not observe CoVac-1-associated grade 4 or significant side effects. The anticipated formation of local granuloma was seen in 94% of study participants. On the other hand, systemic reactogenicity was mainly nonexistent or mild.
On day 28, SARS-CoV-2-selective T cell reactions were generated in 86% of the subjects and oriented to numerous CoVac-1 peptides, with an average of four out of six peptides identified by the patients' T cells. These responses were not influenced by any current SARS-CoV-2 Omicron mutants and were regulated by multipurpose T-helper 1 (Th1) CD4+ T cells. These Th1 cells had positivity for interferon γ (IFN-γ), CD107a, interleukin-2 (IL-2), and tumor necrosis factor (TNF).
According to subgroup analysis, on day 28, those with acquired B-cell deficit had superior response rates and percentages of CoVac-1-triggered T cells than individuals with congenital B-cell deficiency. There was no discernible variance in the frequency and strength of CoVac-1-generated T-cell responses among cancer patients receiving anti-CD20 treatment and those who did not.
Further, in B-cell lacking individuals and immunocompetent seroconverted/non-seroconverted COVID-19 recovered subjects, CoVac-1-generated T-cell responses outperformed S-specific T-cell responses following vaccination with messenger ribonucleic acid (mRNA) vaccines. CoVac-1 was also able to enhance pre-existing S-specific T-cell responses.
None of the participants demonstrated any humoral immune response to SARS-CoV-2 at research inclusion, despite receiving more than two doses of approved COVID-19 vaccines. However, low-degree SARS-CoV-2 anti-S immunoglobulin G (IgG) antibodies generation was noticed in patients upon a single CoVac-1 dose administration, despite continually negative results in serial SARS-CoV-2 polymerase chain reactions (PCRs).
Conclusions
The present study reported the immunogenicity, reactogenicity, and safety of CoVac-1 in the at-risk cohorts with acquired or congenital B-cell deficiency. Even in this drastically immunocompromised sample population, this investigation verified the excellent safety record and demonstrated robust de novo activation of T-cell responses following a single CoVac-1 dose.
The authors concluded that with an outstanding safety characteristic, CoVac-1 elicits broad and robust T-cell responses in individuals with antibody/B cell deficit, irrespective of existing SARS-CoV-2 VOCs. The current data support moving forward of CoVac-1 to a pivotal Phase 2/3 effectiveness and safety study. A long-term Phase 2/3 efficacy research using CoVac-1 is now being prepared to determine which phenotypes and frequencies of T cells are needed to help tackle COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Juliane Walz, Jonas Heitmann, Claudia Tandler et al. Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency, 02 June 2022, PREPRINT (Version 1) available at Research Square, https://doi.org/10.21203/rs.3.rs-1693355/v1, https://www.researchsquare.com/article/rs-1693355/v1
- Peer reviewed and published scientific report.
Heitmann, Jonas S., Tatjana Bilich, Claudia Tandler, Annika Nelde, Yacine Maringer, Maddalena Marconato, Julia Reusch, et al. 2021. “A COVID-19 Peptide Vaccine for the Induction of SARS-CoV-2 T Cell Immunity.” Nature, November. https://doi.org/10.1038/s41586-021-04232-5. https://www.nature.com/articles/s41586-021-04232-5.