Introduction
Coronaviruses (CoVs) have a broad host range, which has led to a widely divergent array of virus structures and a high potential for zoonosis. The CoVs found to cause human disease are mostly derived from rodents or bats.
Four endemic seasonal human CoVs were circulating long before the emergence of the severe acute respiratory syndrome (SARS)-CoV, Middle East Respiratory Syndrome (MERS)-CoV, and SARS-CoV-2. These early endemic CoVs cause about a third of common colds in humans, two of which are closely related to rodent-borne CoVs.
Thus, rodents are likely CoV reservoirs and are capable of spillover events into humans that can ultimately trigger human infections.
Bank voles are commonly found in Europe and carry multiple zoonotic pathogens, including Puumala orthohantavirus and Francisella tularensis. Both alpha- and beta-CoVs have been detected at various times in these animals.
In the current study, researchers report on the discovery of two complete Grimso virus sequences and their phylogenetic relationship to other rodents CoVs. These sequences were obtained from a three-year sampling of bank voles at a single site in Grimso, Sweden between 2015 and 2017.
Study findings
The researchers initially used a customized polymerase chain reaction (PCR) assay to screen approximately 270 bank voles. This yielded nine partial sequences that shared nucleotides and amino acids at 98-100% and 94-100% identity, respectively. The same sequences were less than 60% identical to other beta-CoVs in rodents at the nucleotide level and less than 50% at the amino acid level.
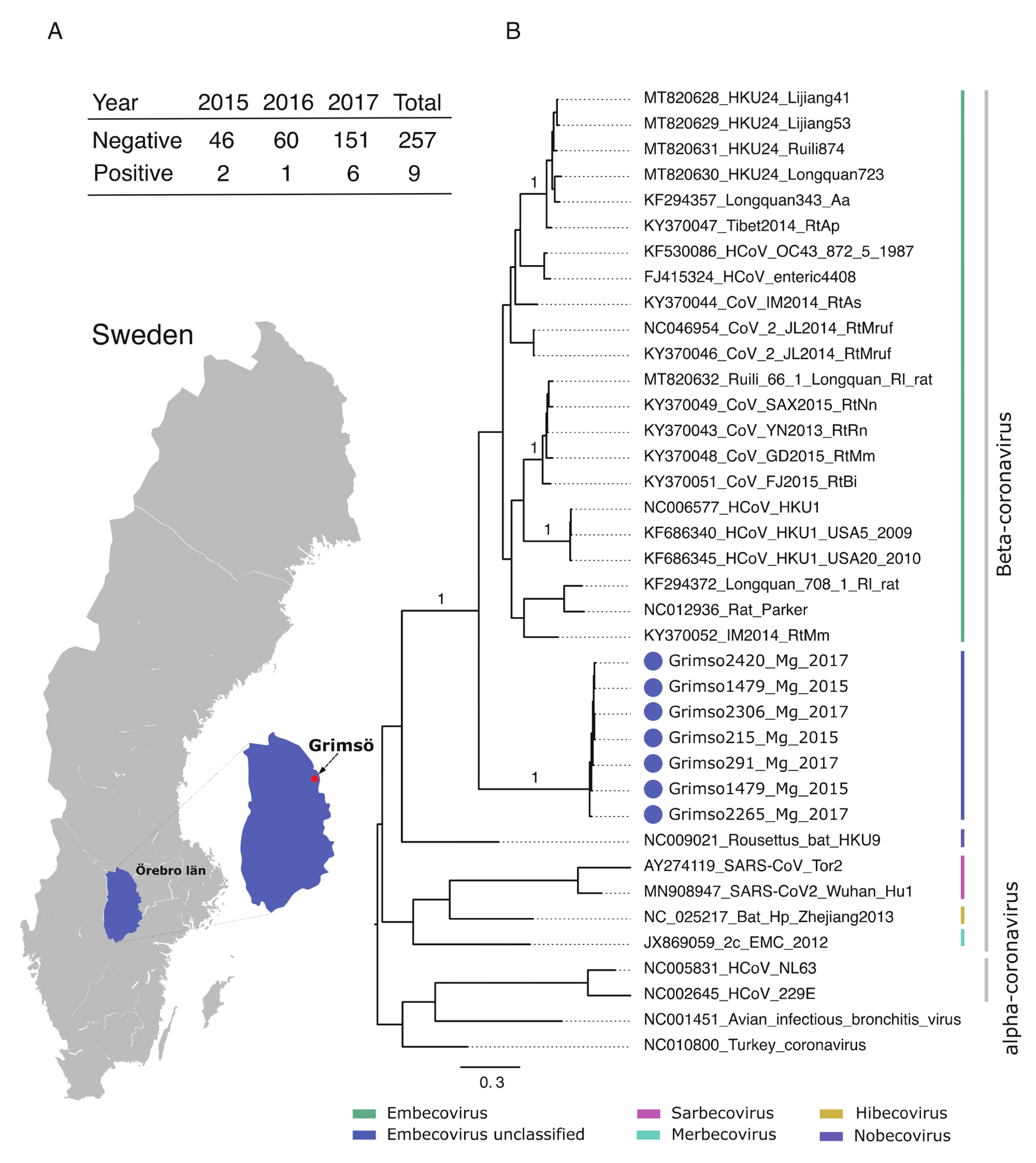
(A) Geographic map showing the province Örebro, Sweden and the site of sampling (Grimsö) where bank voles were captured. Table demonstrating the prevalence of Grimso virus from 2015 to 2017. (B) MrBayes midpoint root tree based on the 252 nt of the spike gene. The scale bar indicates the nt substitution per site. The numbers above the branches indicate the posterior probability. Grimso virus samples are highlighted in blue.
Further analysis indicated that these partial sequences originated from a separate phylogenetic group that formed part of the rodent Embecoviruses.
Using two of these sequences, the researchers obtained a complete sequence with seven regions including the spike, envelope, nucleocapsid, and membrane proteins. These sequences were confirmed to share less than 80% and 67% of nucleotides and amino acid sequences, respectively, with other rodent CoVs. This demonstrates its divergence from other known rodent CoVs.
When compared across 101 CoV genome sequences, the ribonucleic acid (RNA)-dependent RNA polymerase (RdRp) enzyme sequence consisted of three endemic alpha- and beta-CoV species in bank voles. These CoVs belong to Luchacovirus and Embecovirus from genera Alphacoronavirus and Betacoronavirus, respectively. However, common receptor sequences were not identified, except in the S2 domain of the spike protein.
Implications
The current study discusses the discovery of a novel beta-CoV in Swedish bank voles and presents its full sequence for the first time. The failure to detect this virus using a published pan-CoV PCR test is traceable to the highly divergent RNA sequence of the Grimso virus. The use of specific spike gene primers in the customized PCR used in the present study yielded several samples over the three-year study period.
The two complete sequences obtained in this study showed almost 3.5% non-identity, which amounted to about 1,340 differences at the nucleotide level. This exceeds the expected rate of nucleotide substitution over three years. Either several strains of the Grimso virus are co-circulating in this rodent species, or the true reservoir of this virus includes other species that regularly transmit it to bank voles.
The prevalence of the Grimso virus in this rodent species was about 3.4%, thus suggesting that this animal is a suitable host for the virus. As a result, the Grimso virus presents a potential zoonotic host for spillover events between bank voles and human beings.
Bank voles have been shown to carry several different CoVs, including some that are closely related to the Grimso virus, across a broad swath of European territory. Furthermore, since recombination events and cross-species transmission events have often been recorded, there remains an urgent need to conduct additional studies to map and monitor this virus.
Journal reference:
- Wasberg, A., Raghwani, J., Li, J., et al. (2022). Discovery of a Novel Coronavirus in Swedish Bank Voles (Myodes glareolus). Viruses. doi:10.3390/v14061205.