Early diagnosis of infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causal agent of the coronavirus disease 2019 (COVID-19) pandemic, is important to restrict its transmission. In this context, a reliable point-of-care testing system is urgently needed. Some of the necessary features for mass testing of COVID-19 include short detection time, low-cost procedure, and simple sample collection method.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The reverse transcription-polymerase chain reaction (RT-PCR) assay is the gold standard for COVID-19 detection. However, this technique cannot be used for rapid and mass testing, as RT-PCR requires skilled personnel to collect test samples from the infected person and perform this assay.
Thus, the development of an exhaled breath analysis device for the rapid detection of COVID-19 could be extremely convenient. This type of device could be highly beneficial because of its simple sample collection method, non-invasive nature, reusability, and ability to deliver results within a short interval.
Interestingly, previous studies have reported a distinct scent from COVID-19 patients that was easily detected by dogs. The compound that was detected by the dog was further analyzed through gas chromatography-ion mobility spectrometry.
Breath samples can be used to detect SARS-CoV-2 infection by determining the presence of volatile organic compounds (VOCs) in the sample. The exhaled breath of a COVID-19 patient contains specific VOCs that arise due to altered metabolic reactions or changes in the microbiota of the lungs during infection.
The exhaled breath, which contains various gases including VOCs, can be acquired by an electronic nose device. Unlike RT-PCR tests, an electronic nose device can easily detect SARS-CoV-2 infection during its varied developmental phases.
Several studies have reported the development of nanoparticle gas sensors, carbon nanotube sensors, and metal oxide gas sensors for the selective detection of VOCs. Previous studies have also shown that exhaled breath analysis techniques can detect viruses, such as influenza and human rhinoviruses, through the analysis of exhaled VOCs using an electronic nose device.
There are several concerns regarding COVID-19 detection through an electric nose breath analyzer, such as the limited repeatability of infection detection through this approach. Additionally, these devices cannot be applied in varied environmental conditions for breath sample collection and analysis.
About the study
In a recent study under review in the Scientific Reports journal and published on the preprint server Research Square*, researchers discuss the development of an electronic nose device for the detection of VOCs by using commercial gas sensors, which require low energy for their operation at high temperatures. Herein, researchers conducted clinical studies in the northern part of Poland to assess the efficacy of the device.
In the current study, scientists performed the experimental studies in hospital wards that were dedicated to treating SARS-CoV-2 infected patients during the third COVID-19 wave in Poland, which was between March and July 2021. Breath samples were obtained from the University Center of Maritime and Tropical Medicine, Poland, and subsequently analyzed by the newly developed electronic nose device.
A total of 56 breath samples, which consisted of 33 samples obtained from severely infected COVID-19 patients, 17 from healthy controls, and six from ambient air, were used for the current study. All samples were collected in the morning after the study participants brushed their teeth but before having breakfast. This measure was taken to reduce contaminated breath sampling.
About 130 mL of breath sample was collected by a BioVOC™ breath sampler from all study participants breathing in the same atmosphere of the hospital ward.
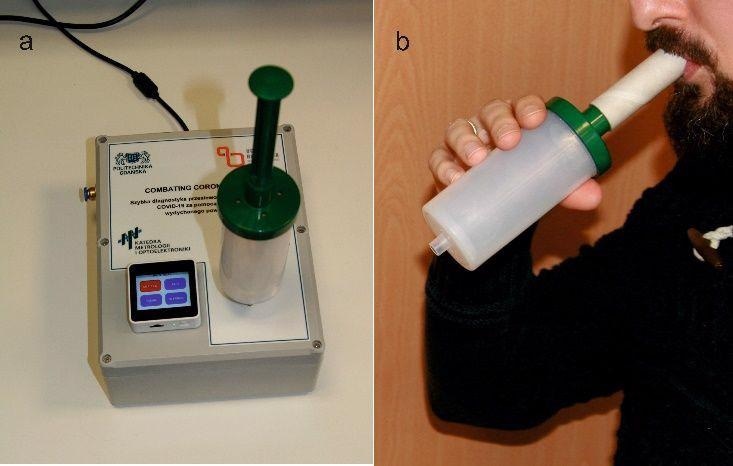
Developed electronic nose analyzing the sample of exhaled breath by using the end-tidal part the final wave, and (b) illustration of collecting the breath sample with the BioVOCTM.
Study findings
Breath samples obtained from COVID-19 patients exhibited higher humidity levels as compared to controls. Notably, the electronic nose device used here provided a better COVID-19 detection rate for the elderly group as to younger patients. In fact, the device provided a detection accuracy as low as 80% in some younger patients.
The device described here could be used for assessing the efficacy of a particular medical treatment or for mass pre-screening. One of the challenges associated with this device is its limited accuracy when used outdoors; thus, future studies should focus on enhancing its accuracy and improving its resistance to fluctuating environmental factors.
Future research
Taken together, the results of the current study strongly suggest that the use of commercial gas sensors is favorable for the identification of VOCs in breath samples. Therefore, the device and methodology used in the current study could be applied in practice for the detection of COVID-19.
In the future, more research is needed to determine why repeated calibration of the device must be performed during its operation. Additionally, a more efficient and faster procedure for cleaning the gas sensor is required.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kwiatkowski, A., Borys, S., Sikorska, K., et al. (2022) Detecting COVID-19 from Exhaled Breath – Clinical Studies. Research Square. doi:10.21203/rs.3.rs-1655727/v1. https://www.researchsquare.com/article/rs-1655727/v1.
- Peer reviewed and published scientific report.
Kwiatkowski, Andrzej, Sebastian Borys, Katarzyna Sikorska, Katarzyna Drozdowska, and Janusz M. Smulko. 2022. “Clinical Studies of Detecting COVID-19 from Exhaled Breath with Electronic Nose.” Scientific Reports 12 (1). https://doi.org/10.1038/s41598-022-20534-8. https://www.nature.com/articles/s41598-022-20534-8.