Respiratory illnesses, like influenza and coronavirus disease 2019 (COVID-19), cause significant mortality and morbidity, primarily affecting the elderly. Fever is a common characteristic of COVID-19 and influenza, although its physiological significance in viral infection resistance among hosts is unclear. The relevance of additional age-linked alterations in host variables other than impaired type I interferons (IFNs) signaling in influenza virus infection susceptibility is unknown.
Multiple studies showed that the gut microbiota makeup in both animals and humans varies with age. In addition, as people get older, their average body temperature drops. Although it is becoming increasingly clear that gut microbiota plus their metabolites are critical for influenza infection protection, the impacts of core body temperature upon host defense against influenza virus infection are mainly uncertain.
The current study's authors previously established that after intranasal treatment with a sublethal 30 pfu dosage of influenza virus, mice exposed to a high ambient 36 °C temperature exhibit impaired virus-selective CD8+ T cell reactions and antibody generation. The outside temperature's impact on host resistance to challenge with a deadly influenza virus, on the other hand, remains unknown.
About the study
In the present study, the scientists investigated the impact of core and outside body temperature on host resistance against influenza infections. They tested host resistance to SARS-CoV-2 or influenza virus infection by exposing mice to a high ambient temperature of 36°C.
The mice were housed at 36, 22, or 4°C for a week preceding infection with influenza virus. High heat-, cold-, and room temperature (RT)-exposed mice were intranasally infected with a mouse-adapted influenza A virus strain A/Puerto Rico/8/1934 (PR8) and maintained at 36, 22, or 4°C for the whole duration of the assessments to explore the effect of core body temperature in influenza virus infection protection.
The authors confined mice at 36, 34, 28, or 22 °C before infecting them with the influenza virus to establish the minimum core body temperature needed for protection from infection. They infected antibiotics (Abx)-treated or low fiber (LF)-fed mice exposed to high heat to see if gut microbial metabolites or microbiota were required to improve host resistance to influenza virus infection.
The investigators conducted gas chromatography-mass spectrometry (GC-MS), capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS)-centered metabolome assessment of serum, cecal contents, and livers of naive mice kept at 36, 22, or 4°C for a week to evaluate the mechanisms underpinning gut microbiota-stemmed metabolite triggered host resistance in high heat-exposed mice to influenza infection.
Moreover, the team examined the inhibitory impact of bile acids on SARS-CoV-2 infection. In the absence or presence of bile acids, they infected VeroE6/TMPRSS2 cells with SARS-CoV-2. Further, the scientists examined the link between bile acid titers in COVID-19 patients' plasma and illness severity.
Results
The study results show that exposing mice to high ambient 36 °C temperature enhances host resistance against viral pathogens such as influenza and SARS-CoV-2. High-heat-exposed mice raise their basal body temperature above 38°C, allowing the production of more bile acid in a gut microbiota-reliant way in the intestine and serum.
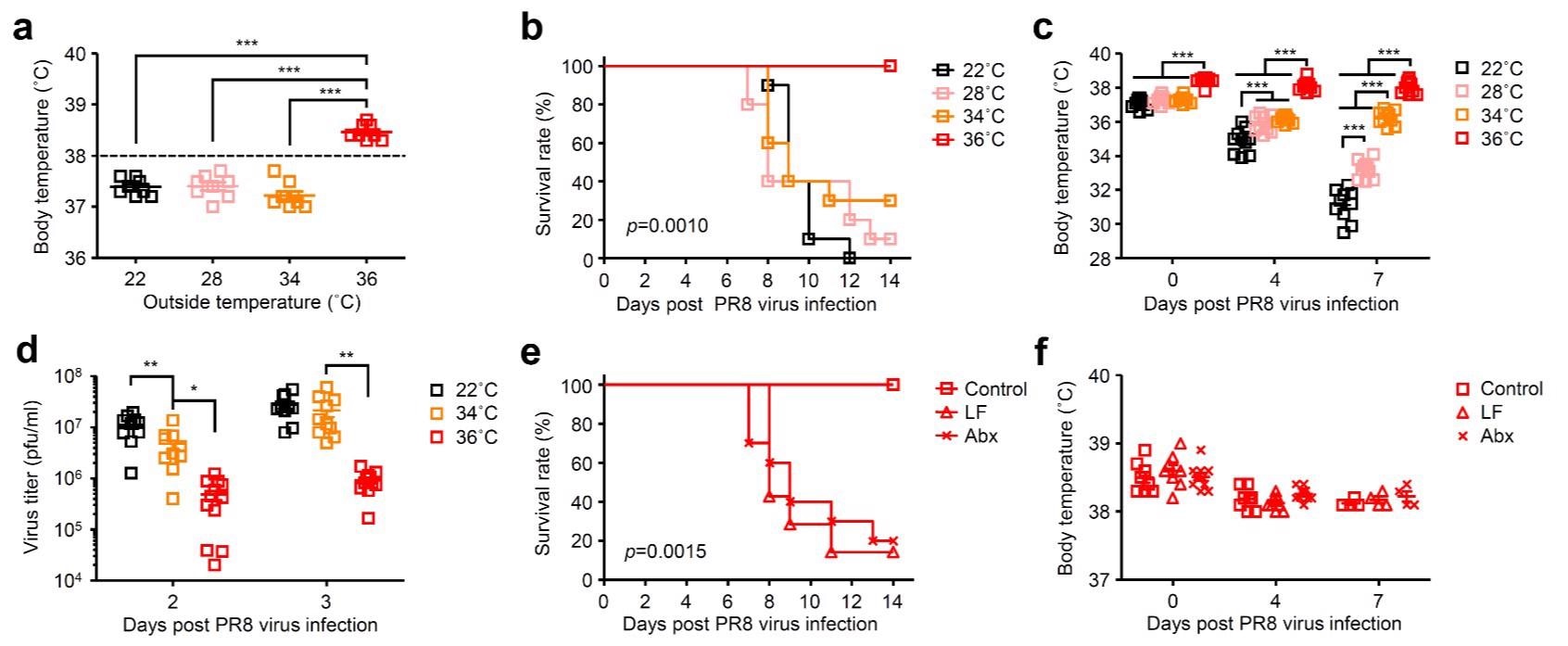 High body temperature increases gut microbiota-dependent host resistance to influenza virus infection. Mice were kept at 22, 28, 34, or 36 °C for 7 d before influenza virus infection and throughout infection. a, Body temperature of naïve mice kept at 22, 28, 34, or 36 °C were measured. b-d, Mice kept at 22, 28, 34, or 36 °C were infected intranasally with 1,000 pfu of influenza virus. Mortality (b), core body temperatures (c), and virus titer in the lung wash (d) were measured on indicated days after challenge. e, f, LF-fed, Abx-treated, and control mice kept at 36 °C were infected intranasally with 1,000 pfu of influenza virus. Mortality (e) and core body temperatures (f) were measured on indicated days after challenge.
High body temperature increases gut microbiota-dependent host resistance to influenza virus infection. Mice were kept at 22, 28, 34, or 36 °C for 7 d before influenza virus infection and throughout infection. a, Body temperature of naïve mice kept at 22, 28, 34, or 36 °C were measured. b-d, Mice kept at 22, 28, 34, or 36 °C were infected intranasally with 1,000 pfu of influenza virus. Mortality (b), core body temperatures (c), and virus titer in the lung wash (d) were measured on indicated days after challenge. e, f, LF-fed, Abx-treated, and control mice kept at 36 °C were infected intranasally with 1,000 pfu of influenza virus. Mortality (e) and core body temperatures (f) were measured on indicated days after challenge.
By restricting viral replication and neutrophil-based tissue damage, the gut microbiota-stemmed deoxycholic acid (DCA) plus its plasma membrane-attached receptor Takeda G-protein-coupled receptor 5 (TGR5) expression increases host resistance to infection with influenza virus.
Furthermore, the Syrian hamster was protected from severe SARS-CoV-2 infection by the DCA plus its nuclear farnesoid X receptor (FXR) agonist. Additionally, compared to the minor sickness group, the plasma of SARS-CoV-2-infected individuals with moderate 1 or 2 diseases had lower levels of specific bile acids.
Overall, the current findings reveal an unanticipated pathway by which SARS-CoV-2 and influenza virus resistance was increased among hosts in a gut microbiota-reliant way via a high fever induced by the virus.
Conclusions
The study findings depicted a previously unknown relationship between host viral infection resistance, gut microbial action, and core body temperature. The researchers discovered that exposing mice to a high ambient 36 °C temperature raises body temperature and enhances host resistance to SARS-CoV-2 or influenza virus infection. Moreover, the study found that activation of gut microbiota based on high body temperature elevates bile acid concentrations in the intestine and serum, which reduces virus replication and harmful inflammatory responses after SARS-CoV-2 and influenza virus infection.
The current article notably adds to the comprehension of how host resistance to SARS-CoV-2 and influenza infection was heightened by core body temperature. The present inference that specific bile acids were diminished in the plasma of individuals with moderate 1/2 COVID-19 might elucidate the diversity in clinical illness manifestation among humans and assist COVID-19 outcome mitigation strategies.

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.