In a recent study under review at the Scientific Reports journal and currently posted to the Research Square* preprint server, researchers synthesized and evaluated the antitumoral (against several cancer cell lines) and antiviral [against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] properties of 3-spiro-indolin-2-ones.
Several spiro-indole compounds obtained from natural sources (organisms) with substantial biological properties are recognized such as maremycin G and F, spirotryprostatins A and B, strychnofoline, and surugatoxin. The compounds have demonstrated antimitotic activity, and in addition, several chemically synthesized spiro-indole analog molecules have exhibited significant cholinesterase inhibitory, antitumor, and antimicrobial properties.
 Study: Spiro-3-indolin-2-ones: Synthesis, biological properties and computational studies. Image Credit: Emin Kuliyev / Shutterstock
Study: Spiro-3-indolin-2-ones: Synthesis, biological properties and computational studies. Image Credit: Emin Kuliyev / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers investigated the anti-SARS-CoV-2 and antitumoral properties of the 3-spiro-indolin-2-ones group of spiro-indole compounds.
First, the spiro-3-indolin-2-ones 6a‒o compounds were chemically synthesized and the structures of the synthesized spiro-indole compounds were characterized by X-ray single crystallography and several spectroscopy methods [infra-red (IR) spectroscopy, proton nuclear magnetic resonance (1H-NMR) spectroscopy, and carbon 13 NMR (13C-NMR) spectroscopy].
Subsequently, the antiproliferation properties of the synthesized compounds were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays and several cancer cell lines [PaCa-2 (pancreatic cancer), A431 (epidermoid carcinoma), HCT116 (colorectal carcinoma), Michigan cancer foundation-7 (MCF7, breast cancer), and RPE1 (retinal pigment epithelial 1 cells (RPE1)]. Anti-neoplastic drugs, viz. sunitinib and 5-fluorouracil (5-FU) were used for reference.
Further, cell cycle studies were performed by flow cytometry (FC) analysis of the compounds that demonstrated the highest potency against the proliferation of MCF7 cells in the MTT assays based on their half-maximal inhibitory concentration (IC50) values. The % deoxyribonucleic acid (DNA) content of the selected compounds (6l and 6m), and controls were determined during the resting phase (G0)-gap 1 phase (G1), synthesis (S), and gap 2 or growth 2 phase (G2)/mitosis (M) phases of the cell cycle studies using MCF7 cells. In addition, the % apoptosis and necrosis by the 6l and 6m compounds and controls using MCF7 cells were assessed.
Furthermore, the inhibition of the epidermal growth factor receptor (EFGR)/vascular endothelial growth factor receptor 2 (VEGFR-2) by the compounds was assessed using the western blot analysis and IC50 values obtained for the compounds. The anti-SARS-CoV-2 properties of the compounds were assessed using Vero-E6 cells and chloroquine, favipiravir, and hydroxychloroquine, and were used for reference. The inhibition of cholinesterase was assessed and molecular QSAR (quantitative structure-activity relationship) modeling studies were performed.
Results
The spiro-indole compounds were synthesized by azomethine dipolar cycloaddition to the exocyclic olefinic linkage of 3,5-bis(arylidene)-N-sulfonyl-4- piperidones. The 1-(alkylsulfonyl)-3,5-bis(ylidene)-piperidin-4-ones were obtained by dehydrohalogenation of a sulfonyl chloride with 3,5-bis(ylidene)-4-piperidinones in dry tetrahydrofuran (THF) in the presence of triethylamine (TEA). Azomethine ylide was obtained by isatin and sarcosine 5 condensations in situ.
Analysis of the compounds’ chemical structures by spectroscopy and X-ray single crystallography showed similar pyrrolidine-piperidine geometry of the 6b, 6c, and 6d compounds based on their angles of torsion. The 6h compound showed a >20° deviation for the 4-5-6-1 angle and also contained ethanol. In the MTT assays conducted using MCF7 cells, compounds 6m (IC50 = 3.6 µM) and 6l (IC50 = 3.9 µM) demonstrated the highest antiproliferation potency comparable with the reference drugs. Higher antiproliferation effects corresponded with lower induction (‒I) effects.
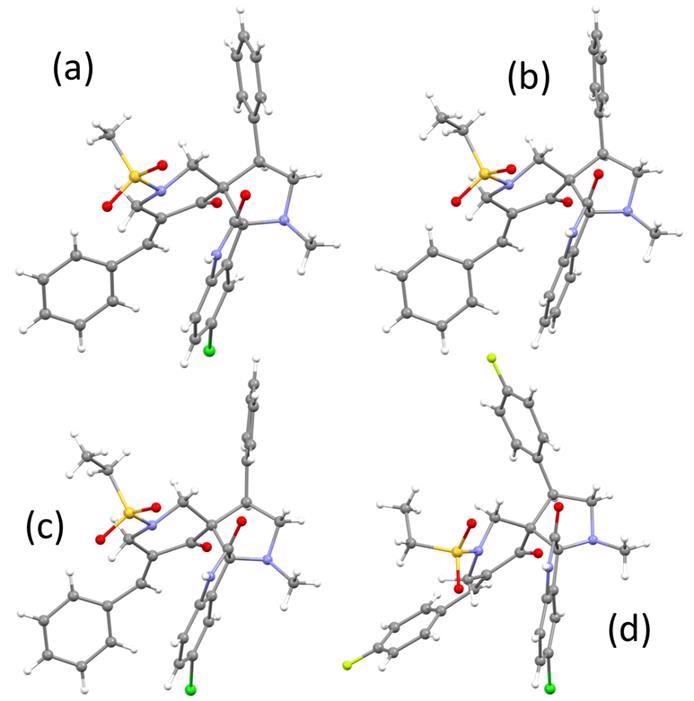
The molecular structures of (a) 6b, (b) 6c (c) 6d and (d) 6h.
All the spiro-indole compounds containing chloroindolinyl (except 6o) showed increased anti-HCT116 properties, and the 6m compound was most effective than the reference drugs with IC50 values of 3.2, 20.4, and 9.7 µM for 6m, 5-FU, and sunitinib, respectively. Similar results were obtained using A431 cells, with the highest potency demonstrated by 6m (IC50 = 2.4 µM) which was greater than that of 5-FU by 9.6-fold and comparable to the 6k compound (IC50 = 2.9 µM). Fluorophenyl-containing compounds showed lower antiproliferation potency in A431 cells.
Compounds 6k and 6j were most potent against PaCa-2 cell proliferation (IC50 = 8.8 µM for both compounds). The selectivity index (SI) of the compounds 6m,6k, 6d, and 6h were 4.1 to 6.1, 1.7, >3.8, and >3.6, respectively, against RPE1 cells and confirmed non-malignant cell safety.
In the cell cycle studies using MCF7 cells, the % DNA content was higher for compounds 6l and 6m compared to controls in all phases. In comparison to 6m, 6l had higher %DNA in the G0-G1 (6l vs. 6m was 66% vs. 63%) and G2/M (6l vs. 6m 10 vs. 2.4) phases, whereas 6m induced more necrosis and total apoptosis (6m vs. 6l was 43 vs. 38) of cancer cells.
Compounds 6c and 6n demonstrated the highest anti-EFGR efficacy, and compounds 6k and 6h were most effective against VEGFR-2. Compound 6f showed the highest anti-SARS-CoV-2 efficacy (IC50 = 7.7 µM), which was 3.3-fold and 4.8-fold higher than chloroquine and hydroxychloroquine. Compounds containing ethylpiperidone were less potent than fluorophenyl-containing ones against SARS-CoV-2.
Compound 6g showed the most potent inhibition of acetylcholinesterase (IC50 = 2.5 μM) and butyrylcholinesterase (IC50 = 3.2 μM) and in general, compounds containing fluorophenyl were more potent. QSAR modeling studies showed that the antiproliferative potency of compounds was dependent on their mathematical coefficient and descriptor values.
Overall, the study findings highlighted the antitumoral and anti-SARS-CoV-2 efficacy of 3-spiro-indolin-2-ones, which were highest for the bromophenyl-containing compound (6m) compared to the phenyl-containing compounds tested.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Spiro-3-indolin-2-ones: Synthesis, biological properties, and computational studies. Nehmedo G. Fawazy, Siva S. Panda, Ahmed Mostafa, Benson M. Kariuki, Mohamed S. Bekheit , Yassmin Moatasim, Omnia Kutkat, Walid Fayad , May A. El-Manawaty, Ahmed A. F. Soliman, Riham A. El-Shiekh, Aladdin M. Srour, Reham F. Barghash, Adel S. Girgis. Research Square preprint 2022, DOI: https://doi.org/10.21203/rs.3.rs-1660054/v1, https://www.researchsquare.com/article/rs-1660054/v1
- Peer reviewed and published scientific report.
Fawazy, Nehmedo G., Siva S. Panda, Ahmed Mostafa, Benson M. Kariuki, Mohamed S. Bekheit, Yassmin Moatasim, Omnia Kutkat, et al. 2022. “Development of Spiro-3-Indolin-2-One Containing Compounds of Antiproliferative and Anti-SARS-CoV-2 Properties.” Scientific Reports 12 (1): 13880. https://doi.org/10.1038/s41598-022-17883-9. https://www.nature.com/articles/s41598-022-17883-9.