In a recent study posted to the bioRxiv* pre-print server, researchers provide the first preclinical evidence of the usefulness of antagonists of G-protein coupled receptor 183 (GPR183), also known as Epstein-Barr virus-induced gene 2 (EBI2), in reducing coronavirus disease 2019 (COVID-19) severity.
 Study: Oxysterols drive inflammation via GPR183 during influenza virus and SARS-CoV-2 infection. Image Credit: Andrii Vodolazhskyi / Shutterstock
Study: Oxysterols drive inflammation via GPR183 during influenza virus and SARS-CoV-2 infection. Image Credit: Andrii Vodolazhskyi / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Infection from influenza A virus (IAV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to the production of oxidized cholesterols, or oxysterols, such as 7α,25-dihydroxycholesterol (7α,25-OHC), which are markers of lung inflammation.
Studies have shown that allergen exposure increase oxysterols in bronchoalveolar lavage fluid (BALF). Likewise, it increases in the sputum of chronic obstructive pulmonary disease (COPD) patients. However, studies have not investigated oxysterols-driven lung inflammation following viral respiratory infections.
GPR183 expressed on macrophages is a key cellular component of the innate and adaptive immune systems. When combined with its oxysterol ligands, GPR183 facilitates the distribution of immune cells to secondary lymphoid organs. Therefore, blocking GPR183 using antagonists could be therapeutically beneficial in reducing COVID-19-related lung inflammation and disease severity.
About the study
In the present study, researchers used two preclinical murine models of IAV and SARS-CoV-2 infection to demonstrate oxysterols-driven GPR183-mediated macrophage infiltration of the lungs, which can be lethal. It can lead to a cytokine storm, severe lung tissue injury, acute respiratory distress syndrome (ARDS), and death following viral infections, including IAV and SARS-CoV-2 infections.
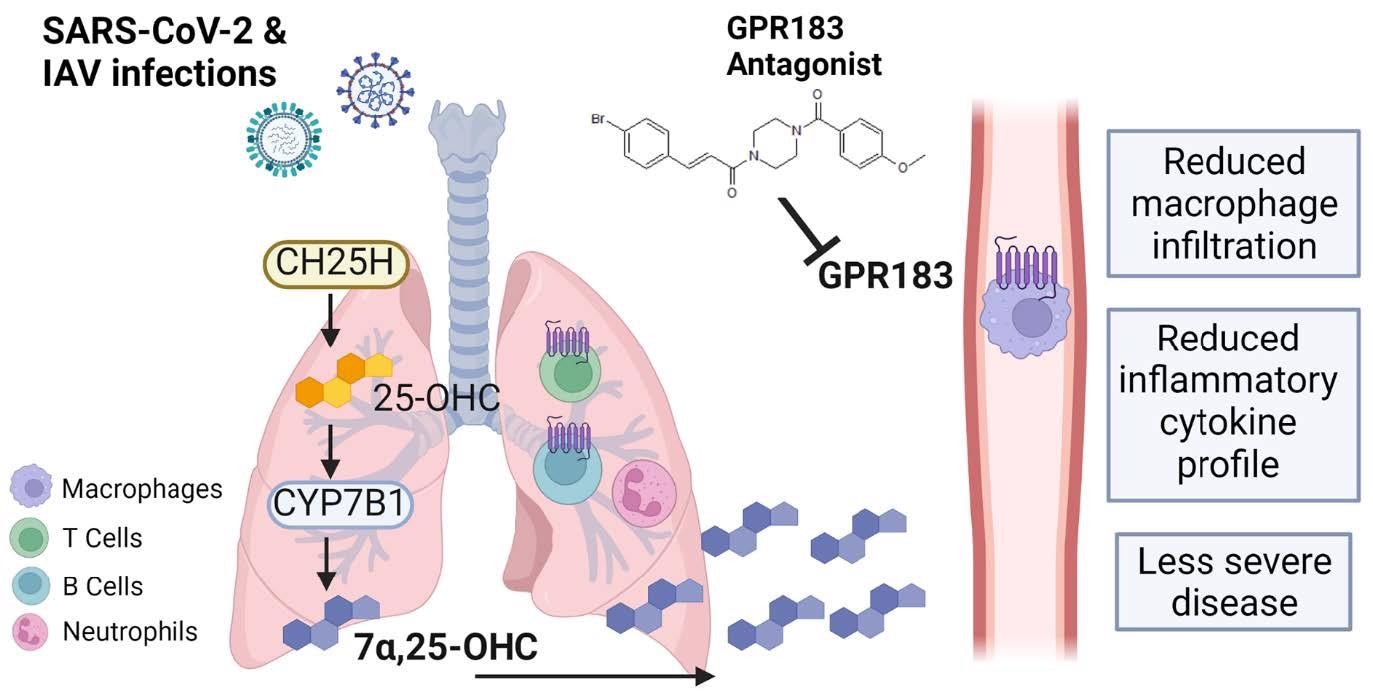
Schematic figure of the role of GPR183 in the immune response to SARS789 CoV-2 and IAV infections. SARS-CoV-2 and IAV infections lead to the upregulation of CH25H and CYP7B1 which results in the production of 7a,25-OHC. This oxysterol chemotactically attracts GPR183-expressing macrophages to the lungs where they produce pro-inflammatory cytokines. Pharmacological inhibition of GPR183 attenuates the infiltration of GPR183-expressing macrophages, leading to reduced production of inflammatory cytokines without negatively affecting antiviral responses.
The team infected mice with IAV and determined the messenger ribonucleic acid (mRNA) expression of oxysterol-producing enzymes, cholesterol 25-hydroxylase (CH25H), and 25HC 7α-hydroxylase (CYP7B1) in their lungs. Likewise, they infected mice with a mouse-adapted SARS-CoV-2 strain by passaging the Beta variant four times in C57BL/6J mice. They also performed experiments on mice genetically deficient in GPR183 (Gpr183-/-).
The team administered synthetic GPR183 antagonist (2E)-3-(4-Bromophenyl)-1-[4-(4-methoxybenzoyl)-1-piperazinyl]-2-propen-1-one (NIBR189) into C57BL/6J mice twice daily starting from 24 h post-infection until the end of the experiment. In addition, they performed flow cytometry analysis on lung single-cell suspensions from C57BL/6J and Gpr183-/- mice treated with NIBR189 and vehicle, respectively. Further, the researchers investigated whether the reduced macrophage infiltration and inflammatory cytokine profile in the lung of the NIBR189-treated mice were associated with altered viral loads based on measurements of nucleocapsid protein (Np) expression.
Study findings
Similar to the IAV infection results, mRNA expression of CH25H and CYP7B1 was significantly upregulated in the lungs of SARS-CoV-2 infected mice. IHC further confirmed these results at the protein level. Also, Gpr183-deficient mice had less severe SARS-CoV-2 infection. Two days post-infection, mice lung homogenates had high concentrations of 7α,25-OHC. Moreover, NIBR189-treated C57BL/6J mice lost less weight, recovered faster, and had significantly reduced macrophage infiltration into the lung at two and five dpi compared to infected C57BL/6J mice receiving vehicle. While the NIBR189 treatment did not affect early IFN responses, IFN responses at five dpi were significantly lower.
The SARS-CoV-2 Np expression was not detected at five dpi when the animals recovered from the infection. However, at the mRNA level, SARS-CoV-2 main protease (Mpro) RNA loads in the lungs of NIBR189-treated mice were significantly lower at five dpi. Overall, the study findings indicated that GPR183 antagonists reduced viral loads, macrophage infiltration, and production of pro-inflammatory cytokines.
Early type I and III interferons (IFNs) are crucial in controlling viral replication during IAV and SARS-CoV-2 infections. Conversely, persistent type I IFN responses can be detrimental to the host and contribute to the development of cytokine storms. Lower pro-inflammatory cytokine production in the NIBR189-treated animals thus indicated good disease outcomes and more effective viral clearance. Notably, unlike 7α,25-OHC, which inhibits SARS-CoV-2 infection in vitro by blocking the virus-host cell membrane fusion, given that it is structurally different from cholesterols, NIBR189 does not disrupt the host cell membrane composition.
Conclusions
The current study demonstrated the dual benefits of the GPR183 antagonist NIBR189. First, NIBR189 reduced macrophage infiltration and inflammatory cytokine production in the lungs of IAV- and SARS-CoV-2-infected animals. However, only in SARS-CoV-2-infected mice NIBR189 significantly improved infection severity by decreasing viral loads. Moreover, short-term use of a GPR183 antagonist during the acute viral infection did not negatively impact antibody responses. Most importantly, a GPR183 antagonist-based therapy could prove effective against newly emerging SARS-CoV-2 variants without further adaption because it targets the host, not the virus. Nevertheless, it requires further investigation into why NIBR189 had no impact on IAV viral loads and whether this was due to pathogen or severity of infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Oxysterols drive inflammation via GPR183 during influenza virus and SARS-CoV-2 infection, Cheng Xiang Foo, Stacey Bartlett, Keng Yih Chew, Minh Dao Ngo, Helle Bielefeldt-Ohmann, Buddhika Jayakody Arachchige, Benjamin Matthews, Sarah Reed, Ran Wang, Matthew J. Sweet, Lucy Burr, Jane E. Sinclair, Rhys Parry, Alexander Khromykh, Kirsty R. Short, Mette M. Rosenkilde, Katharina Ronacher, bioRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.06.14.496214, https://www.biorxiv.org/content/10.1101/2022.06.14.496214v2
- Peer reviewed and published scientific report.
Foo, Cheng Xiang, Stacey Bartlett, Keng Yih Chew, Minh Dao Ngo, Helle Bielefeldt-Ohmann, Buddhika Jayakody Arachchige, Benjamin Matthews, et al. 2022. “GPR183 Antagonism Reduces Macrophage Infiltration in Influenza and SARS-CoV-2 Infection.” European Respiratory Journal, November, 2201306. https://doi.org/10.1183/13993003.01306-2022. https://erj.ersjournals.com/content/61/3/2201306.