The combination of oral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protease inhibitor nirmatrelvir and its pharmacokinetic booster ritonavir was granted Emergency Use Authorization (EUA) in the United States (US) in December 2021 to decrease the risk of progression to severe COVID-19. Likewise, the World Health Organization (WHO) recommended nirmatrelvir plus ritonavir in April 2022 for the individuals at over 10% risk of hospitalization but deferred its use for those vaccinated for COVID-19 and at a lower risk.
Amid the continuous emergence of SARS-CoV-2 lineages with immune-evasion properties, such as Omicron, an improved understanding of the clinical effectiveness of nirmatrelvir plus ritonavir among Omicron-infected vaccinated individuals is needed to inform public health policies.
About the study
In the present study, researchers used integrated healthcare data of Mass General Brigham (MGB) to identify recorded COVID-19 infections between January 1 and May 15, 2022, any subsequent hospitalizations through May 29, 2022, and deaths through June 12, 2022.
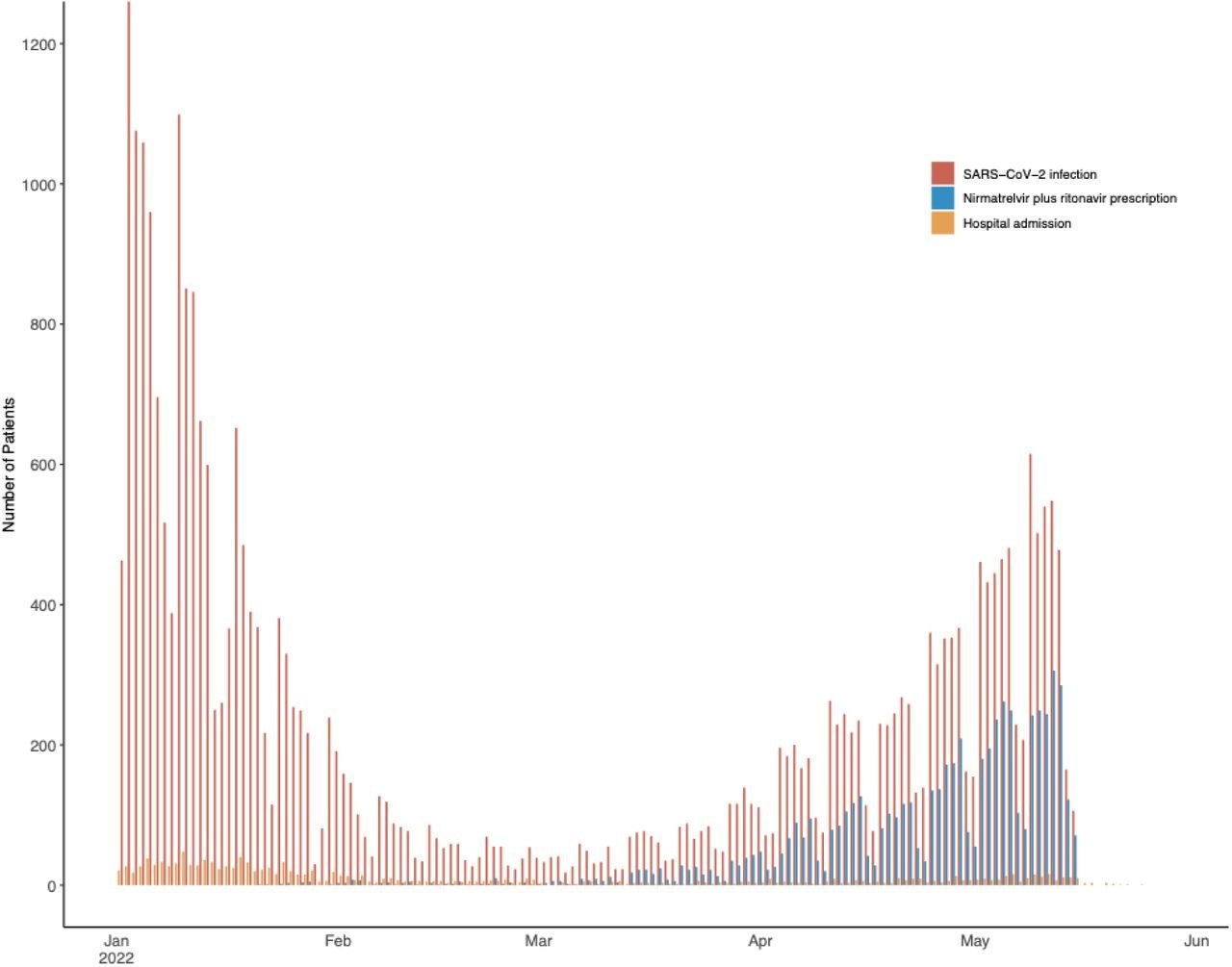
SARS-CoV-2 infection, treatment with nirmatrelvir plus ritonavir, and hospitalization among study patients. Infections and treatment initiation included from January 1 to May 15, 2022. Hospitalizations included through May 29, 2022.
The MGB, a non-profit organization, has been offering excellent COVID-19 care to 1.5 million people annually in Massachusetts and New Hampshire in the US through their academic and community hospitals and a network of ambulatory clinics and community health centers. The MGB utilizes a shared electronic health record (EHR) and electronically prescribes nirmatrelvir plus ritonavir to the highest risk individuals and for all EUA-eligible patients.
The study cohort encompassed individuals 50 years and older with new-onset COVID-19 between January 1 and May 15, 2022, and residing in Massachusetts or New Hampshire. The team obtained each participant's medical condition, date of positive SARS-CoV-2 testing, COVID-19 vaccination and treatment status, medications used at diagnosis, height, weight, race, and ethnicity, and home zip code from their EHRs.
The researchers used recorded medical conditions and age to calculate the monoclonal antibody screening score (MASS). Likewise, they calculated a comorbidity index for each patient that indicated COVID-19 hospitalization risk. The primary study outcome was determining the effectiveness of nirmatrelvir plus ritonavir in reducing the risk of hospitalization in the 14 days following an outpatient COVID-19 diagnosis among all the study participants aged 50 years or more. Further, the researchers compared patients prescribed the drug versus patients not prescribed nirmatrelvir plus ritonavir to estimate the effectiveness of the treatment. Additionally, they reviewed deaths occurring within 28 days of COVID-19 diagnosis.
Study findings
Between January 1 and May 15, 2022, 31,460 MGB patients aged 50 years or older were diagnosed with COVID-19. Of these, 1,138 were only included in the development of testing weights, while the remaining 30,322 outpatients were eligible for nirmatrelvir plus ritonavir treatment. It is noteworthy that nirmatrelvir plus ritonavir was prescribed to 6036 patients who were vaccinated, older, and had higher comorbidity scores. In fact, between January and February 2022, access to nirmatrelvir plus ritonavir was severely limited. Later, a spring wave between April and May 2022 enhanced provider willingness to prescribe nirmatrelvir plus ritonavir.
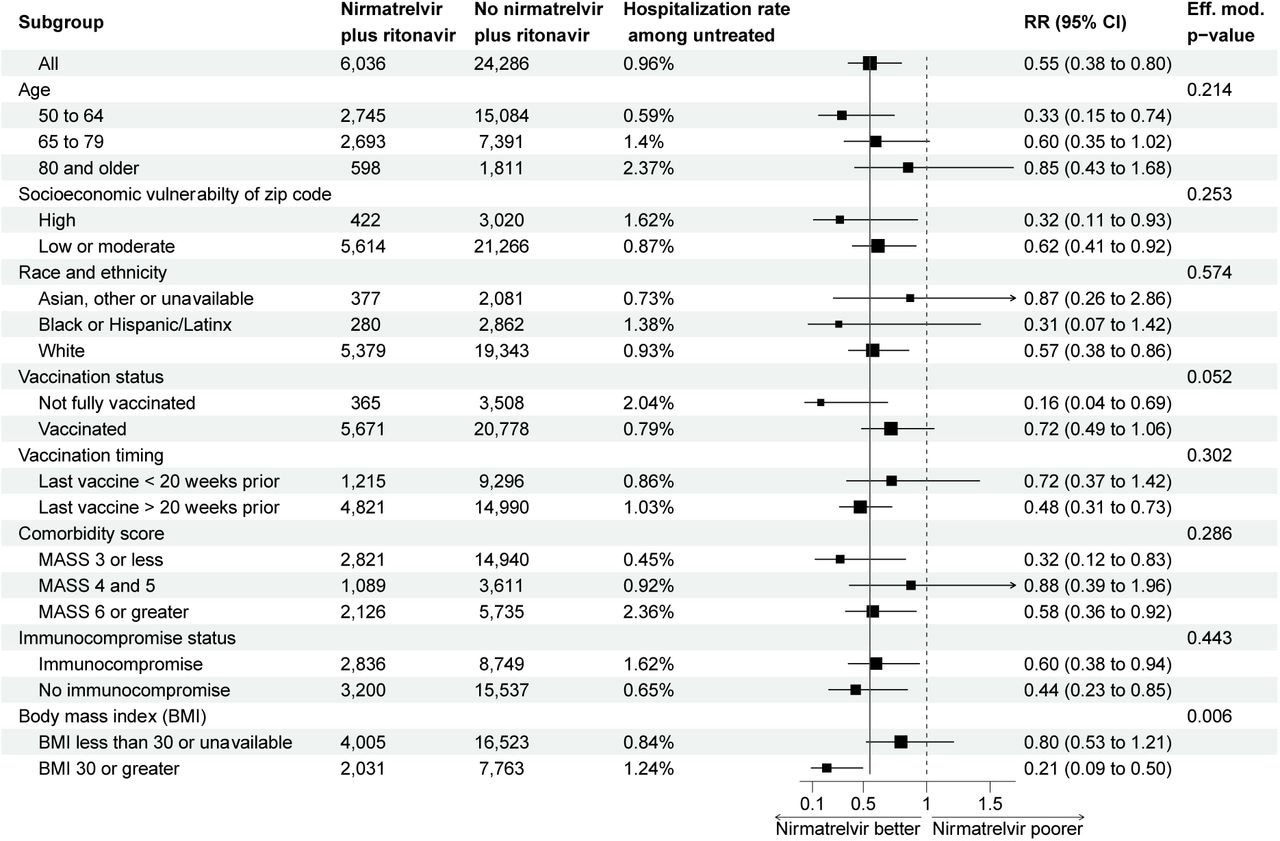
Subgroup analysis of the risk ratio of hospitalization comparing patients prescribed and not prescribed nirmatrelvir plus ritonavir. Estimate and confidence interval calculated from an inverse-probability weighted model performed within in strata. Effect modification p-value calculated from nested models.
The patients residing in socio-economically vulnerable zip codes and belonging to Hispanic or Latin ethnicities were less likely to be prescribed nirmatrelvir plus ritonavir compared to the White people in high-income localities. The authors found that only 40 (0.66%) patients prescribed nirmatrelvir plus ritonavir were hospitalized within 14 days of COVID-19 infection. None of the hospitalizations among nirmatrelvir plus ritonavir recipients were attributable to the rebound syndrome.
The observed risk reduction due to nirmatrelvir/ritonavir was similar across age, comorbidities, and socioeconomic vulnerability; however, it increased protective activity among obese patients with approximate body mass index of 30 kg/m2 and above. Also, nirmatrelvir plus ritonavir appeared more effective among incompletely vaccinated individuals. The authors noted that all 39 deaths within 28 days of COVID-19 diagnosis occurred among patients not prescribed nirmatrelvir plus ritonavir. A staggering 74% of the deaths occurred in vaccinated patients.
Conclusions
Amid an intense Omicron epidemic and high vaccine prevalence among 50 years and older, the current study tested the efficacy of nirmatrelvir plus ritonavir in preventing COVID-19-related hospitalization and death. The hospitalization rate was under 1% (low) among patients diagnosed with COVID-19 as outpatients. The use of nirmatrelvir plus ritonavir further reduced the risk of hospitalization by 45%.
In striking contrast to the EPIC-HR study, 35% of the hospitalizations in the nirmatrelvir plus ritonavir arm of the present study occurred within two days of prescription. Note that the EPIC-HR trial comprised only unvaccinated individuals with a median age under 50 and had a 7% hospitalization rate in the placebo arm compared to the current study.
Overall, nirmatrelvir plus ritonavir consistently protected hospitalization and death despite varying hospitalization rates across groups. Therefore, the authors emphasized continuous assessment of the clinical efficacy of nirmatrelvir plus ritonavir with other therapeutic options as future SARS-CoV-2 variants continue to emerge.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, Scott Dryden-Peterson, Andy Kim, Arthur Y Kim, Ellen C Caniglia, Inga Lennes, Rajesh Patel, Lindsay Gainer, Lisa Dutton, Elizabeth Donahue, Rajesh T Gandhi, Lindsey R Baden, Ann E Woolley, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.06.14.22276393 https://www.medrxiv.org/content/10.1101/2022.06.14.22276393v2
- Peer reviewed and published scientific report.
Dryden-Peterson, Scott, Andy Kim, Arthur Y. Kim, Ellen C. Caniglia, Inga T. Lennes, Rajesh Patel, Lindsay Gainer, et al. 2023. “Nirmatrelvir plus Ritonavir for Early COVID-19 in a Large U.S. Health System.” Annals of Internal Medicine 176 (1): 77–84. https://doi.org/10.7326/m22-2141. https://www.acpjournals.org/doi/10.7326/M22-2141.