The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has impacted the management of cancer patients in many ways. SARS-CoV-2 vaccinations are given priority to cancer patients worldwide due to their poor outcomes during COVID-19.
However, there are not enough trials on the COVID-19 vaccine's safety and immunogenicity, specifically for lung cancer patients receiving chemotherapy. Furthermore, it is unknown how immunogenicity varies according to the type of anticancer medications given to patients. Lastly, it is uncertain how the titers of anti-SARS-CoV-2 receptor-binding domain (RBD) spike (S) protein immunoglobulin G (IgG), i.e., anti-RBD, as determined by two separate immunoassays among lung cancer patients differ.
About the study
The current prospective multicenter cohort investigation explored the safety and immunogenicity of the COVID-19 BNT162b2 messenger ribonucleic acid (mRNA) vaccine among lung cancer patients undergoing anticancer therapy utilizing two immunoassays to quantify anti-RBD titers. The scientists compared the safety and immunogenicity of the BNT162b2 vaccine between lung cancer patients and individuals without cancer experiencing chronic illnesses following the initial and second vaccine doses.
For the research, the team enlisted lung cancer patients undergoing anticancer therapy and non-cancer patients with chronic conditions. All subjects had received two-dose BNT162b2 vaccination at three to four-week intervals (fully-vaccinated). Blood samples were taken pre and post the initial and second vaccine doses and three to five weeks following the second vaccination.
The study comprised 38 non-cancer participants and 55 patients with lung cancer for the vaccine immunogenicity assessments. After eliminating two ineligible patients, 91 patients finished the three blood sampling up to four weeks following the second vaccination. The team evaluated the vaccines' safety and immunogenicity in participants negative for anti-SARS-CoV-2 nucleocapsid (N) antibodies in both assays on 1) two weeks before the initial vaccination (S0), 2) one week after the second vaccination (S1), and 3) four ± one week, i.e., 21 to 25 days, following the second vaccination (S2).
Furthermore, the anti-SARS-CoV-2 S protein S1 subunit RBD antibody concentrations were quantified utilizing the Elecsys anti-SARS-CoV-2 S (Roche Diagnostics) and Architect SARS-CoV-2 IgG II Quant (Abbott Laboratory).
Results
The study results showed that after the first and second dose of BNT162b2 vaccination, lung cancer patients demonstrated a marked hike in the geometric mean titer (GMT) of COVID-19 antibodies, which was substantially lower than the non-cancer patients. The GMTs for cancer relative to non-cancer patients were 30 versus 121 AU/mL on Architect; 4 versus 1.2 U/mL on Elecsys upon initial dose receipt, and after second vaccinations, 1632 versus 3472 AU/mL on Architect; 213 versus 573 A/mL on Elecsys. Indeed, the team discovered that lung cancer patients undergoing anticancer therapy had 98% seropositivity at a threshold value of ≥50 AU/mL.
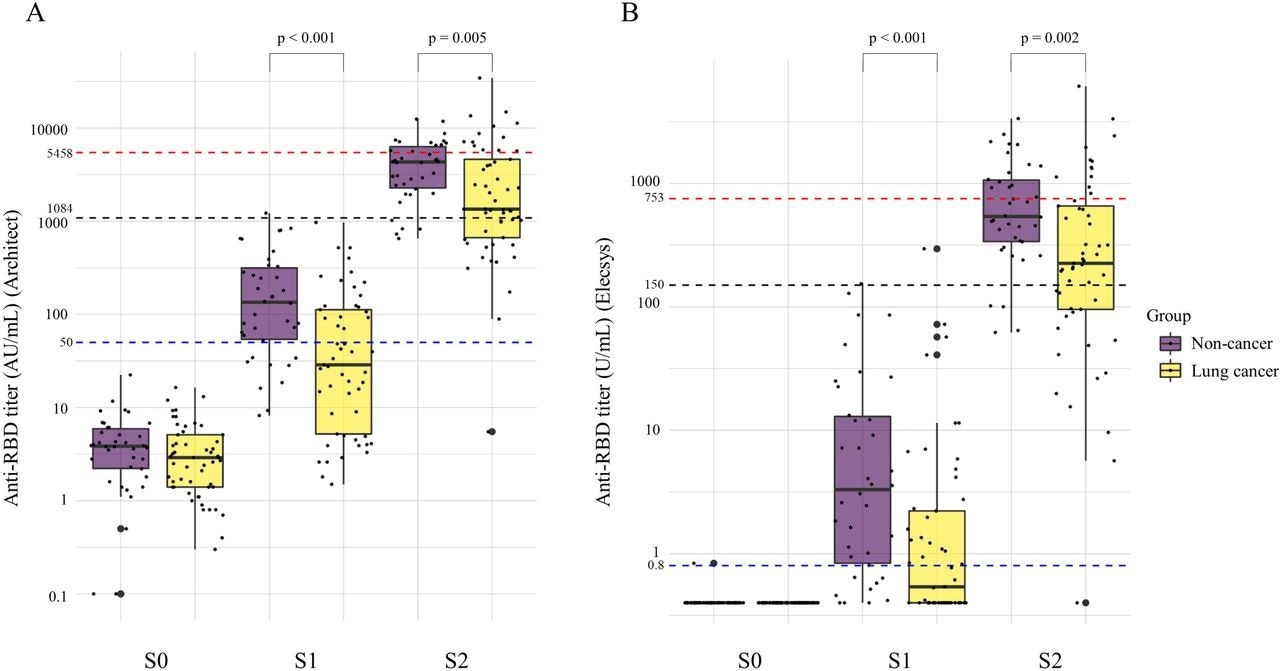
Changes in the anti-RBD antibody titer before vaccination (S0), after first vaccination (S1), and after second vaccination (S2) between non-cancer and lung cancer patients. A Anti-RBD titer on Architect. B Anti-RBD titer on Elecsys. The GMTs of lung cancer patients were significantly lower than those of the non-cancer patients after the first vaccination (30 vs. 121 AU/mL, p<0.001 on Architect; 4.0 vs 1.2 U/mL, p<0.001, on Elecsys) and second vaccination (1632 vs. 3472 AU/mL, p=0.005, on Architect; 213 vs 573 A/mL, p=0.002, on Elecsys). Anti-RBD, anti-severe respiratory syndrome coronavirus-2 receptor-binding domain spike protein IgG S0, within 14 days before the first vaccination; S1, within 7 days before the second vaccination; S2, 4 ± 1 weeks (21–35 days) after the second vaccination Architect, Architect SARS-CoV-2 IgG □ Quant (Abbott Laboratories); Elecsys, Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics)
In the multivariate assessment, the adjusted odds ratio (OR) for seropositivity/seroprotection (≥ 150 AU/mL and ≥ 1,084 AU/mL in Elecsys and Architect assays) by the BNT162b2 vaccination was considerably low in individuals with lung cancer. According to the evaluation of the anticancer therapy types, the modified OR for seroprotection and seropositivity (≥ 150 AU/mL and ≥ 1,084 AU/mL in Elecsys and Architect assays) among lung cancer patients administering cytotoxic drugs was substantially low. These findings suggest that the SARS-CoV-2 vaccine's immunogenicity in lung cancer patients receiving anticancer treatment may not be as good as in non-cancer patients.
The controlled OR for ≥150 AU/mL on Elecsys and ≥1,084 AU/mL on Architect following two vaccine doses tended to drop in lung cancer patients receiving tyrosine kinase inhibitors (TKIs), though the effect was statistically insignificant. Notably, the controlled OR for seroprotection in patients using immune-checkpoint inhibitors (ICIs) were 0.54 for ≥150 U/mL on Elecsys and 0.73 for ≥1,084 U/mL on Architect following the receipt of the second vaccine dose, which did not drop comparable to non-cancer patients.
In addition, after receiving either the initial or second BNT162b2 vaccine dose, lung cancer patients did not exhibit a rise in the frequency of adverse responses compared to non-cancer patients.
Conclusions
Altogether, the authors found that COVID-19 BNT162b2 vaccination resulted in a significantly higher level of antibody titers and an acceptable safety profile in lung cancer patients receiving anticancer treatment in the present study, which may support the use of SARS-CoV-2 vaccination in this patient population. However, compared to patients without cancer, these patients' immunogenicity may be insufficient.
The team recommended that the prioritization of cancer patients for COVID-19 vaccinations should be continued. Additionally, more research is required to evaluate whether greater SARS-CoV-2 vaccine doses, a different type of vaccine, combining various vaccine types, the vaccination timing, or additional vaccine doses improve immunogenicity in cancer patients receiving treatment. Besides, for lung cancer patients receiving anticancer treatment, infection control interventions like universal mask use and social distancing continue to be crucial.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Immunogenicity and safety of COVID-19 vaccine in lung cancer patients receiving anticancer treatment: A prospective multicenter cohort study; Kei Nakashima, Masayuki Ishida, Hiroyuki Matsui, Chihiro Yoshida, Tatsuya Nagai, Minoru Shiraga, Nakaoka Hiroshi, Yoshihito Otsuka, Yu Nakagama, Natsuko Kaku, Yuko Nitahara, Yasutoshi Kido, Hirota Yoshio. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.23.22276536, https://www.medrxiv.org/content/10.1101/2022.06.23.22276536v1
- Peer reviewed and published scientific report.
Nakashima, Kei, Masayuki Ishida, Hiroki Matsui, Chihiro Yoshida, Tatsuya Nagai, Minoru Shiraga, Hiroshi Nakaoka, et al. 2022. “Immunogenicity and Safety of COVID-19 Vaccine in Lung Cancer Patients Receiving Anticancer Treatment: A Prospective Multicenter Cohort Study.” Human Vaccines & Immunotherapeutics 18 (6). https://doi.org/10.1080/21645515.2022.2140549. https://www.tandfonline.com/doi/full/10.1080/21645515.2022.2140549.