
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) therapeutics, which can stop or treat viral transmission, are still urgently needed.
Niclosamide, the Food and Drug Administration (FDA)-authorized oral anthelmintic agent, has broad biological functions, including antibacterial, antiviral, and anticancer effects. Recent reports depicted that niclosamide was a potent inhibitor of COVID-19 in vitro, sparking interest in its application in SARS-CoV-2 infection prevention or treatment.
Nonetheless, there are various potential drawbacks to using niclosamide in COVID-19. These drawbacks included high cellular toxicity, significant polypharmacology, low bioavailability, and uncertain efficacy of niclosamide against arising SARS-CoV-2 variants of concern (VOCs).
About the study
In the current study, the investigators from the University of Michigan employed high-content imaging-based immunofluorescence experiments in two distinct cell models to determine the limitations and the possibility of deploying niclosamide as a SARS-CoV-2 antiviral.
The team expanded the analyses to 33 niclosamide structural analogs to establish a preliminary characterization of the link between structure and activity, potentially assisting the future generation of compounds exhibiting anti-SARS-CoV-2 potency and few off-target impacts. Further, they employed high-content fluorescence imaging to estimate a selectivity index for niclosamide across two distinct cell types to examine niclosamide toxicity. The cell models used were the more physiologically relevant human lung adenocarcinoma cell line, H1437, and VeroE6.
In addition, in VeroE6 cells, the authors assessed niclosamide's effectiveness against the SARS-CoV-2 Alpha (B.1.1.7), wild type (WA1), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants. Finally, they examined the impact of niclosamide therapy on COVID-19 using the morphological characterization of VeroE6 cells infected with Alpha to determine the mechanism of action (MOA).
Results
The study results showed that niclosamide has a dramatically low selectivity index in VeroE6 and H1437 cell lines following 72 hours of compound exposure. This inference indicated that even if a significantly adequate compound exposure for anti-SARS-CoV-2 efficacy occurs in the lungs, niclosamide would probably have a limited therapeutic window in the clinical setting.
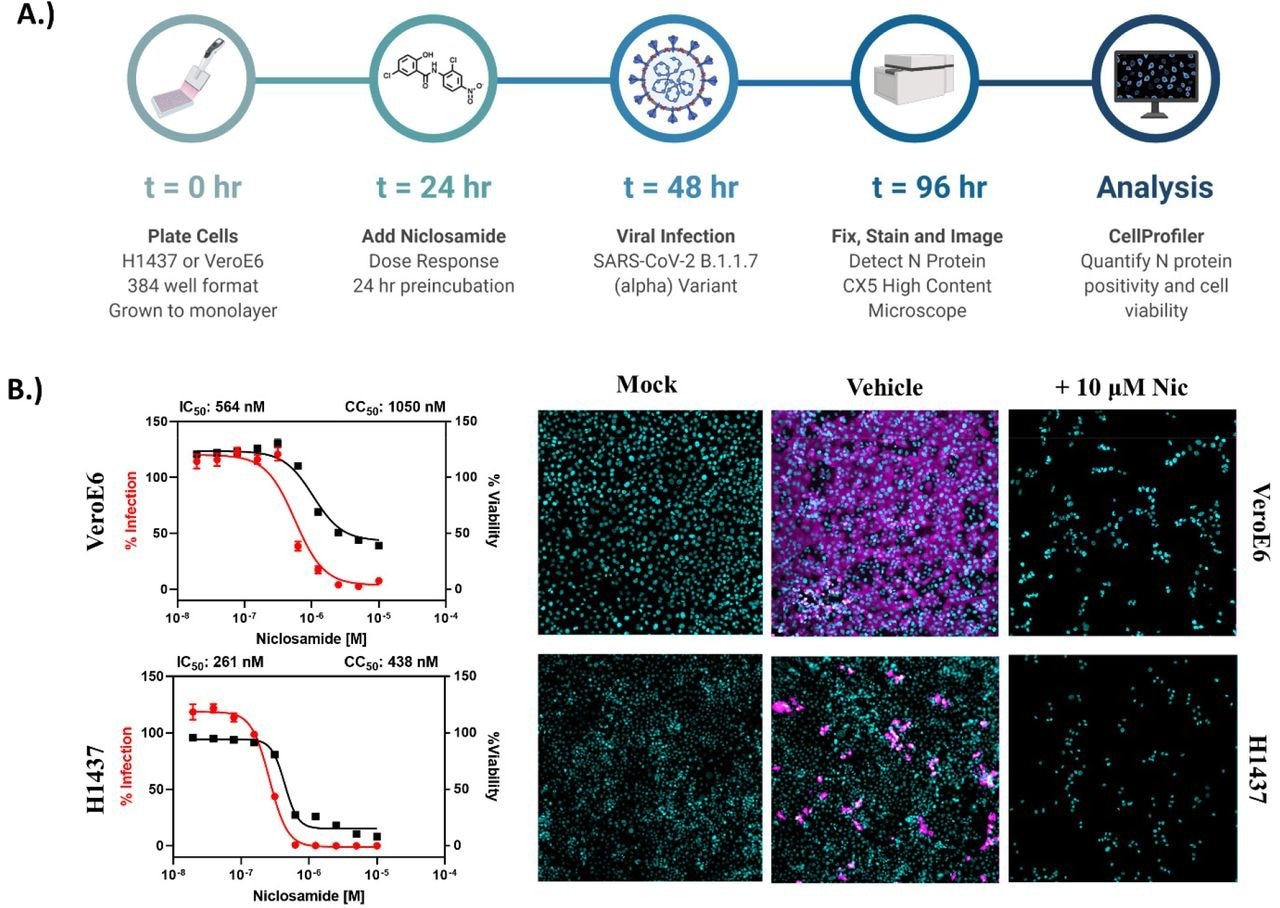
Niclosamide is toxic at antiviral concentrations after long-term exposure. A.) Workflow for high content anti-SARS-CoV-2 bioassay screening to determine infection inhibition and cytotoxicity. B.) 10-point, 2-fold dilution concentration-response curves for VeroE6 and H1437 cells with a starting concentration of 10 μM. VeroE6 cells were infected with SARS-CoV-2 B.1.1.7 variant at a multiplicity of infection (MOI) of 0.1, while H1437 were infected with SARS-CoV-2 WA1 variant at an MOI =1 to achieve optimal infection at 48 hours post-infection. Data points represent mean ± SEM for N=10 replicates per condition. Curve fitting was performed in GraphPad Prism 9.0 using a semi-log 4-parameter variable slope model. Representative overlay images for mock, vehicle and 10 μM Niclosamide treatment (infected) are included (cyan = nuclei, magenta = SARS-CoV-2 N protein).
Despite encouraging preliminary results, the authors demonstrated that niclosamide has a poor in vitro selectivity index for inhibiting SARS-CoV-2 since its antiviral activity coincides with its cytotoxicity. They further show that niclosamide's effectiveness against the SARS-CoV-2 VOCs varies greatly, ranging from 298 nM for the Beta variant to 1664 nM for the wildtype and that it was particularly effective against variants with greater cell-to-cell spread, such as Alpha. According to additional single-cell morphological characterization, niclosamide also hinders the entrance of SARS-CoV-2 and cell-to-cell dissemination through syncytia.
The team discovered eight niclosamide analog compounds (compounds 34, 24, 22, 11, 10, 4, 3, 2) that were effective in both the H1437 and VeroE6 cell models. Four among these (compounds 24, 11, 7, and 2) exhibited reduced cytotoxicities and improved potency than niclosamide in VeroE6. As a result, they believed there was a potential to create niclosamide analogs with better qualities.
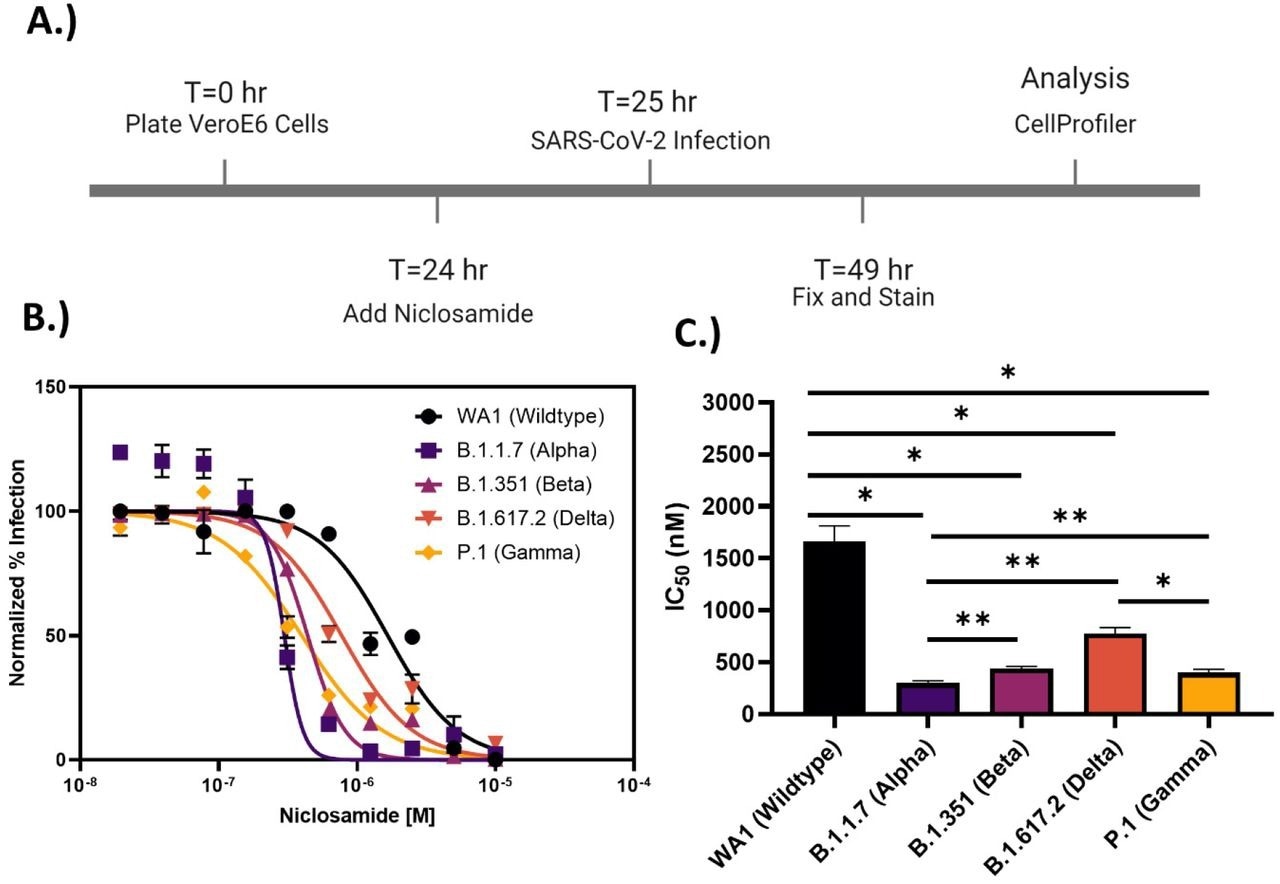
Niclosamide potency is SARS-CoV-2 variant dependent. A.) Assay timeline for 24-hour infection experiment. The assay window was shortened to reduce niclosamide toxicity. B.) 10-point 2-fold concentration-response curves for niclosamide against the different SARS-CoV-2 variants of concern (MOI = 0.1 for each variant) with a top concentration of 10μM. Curves were fitted with GraphPad Prism 9.0 software using a semi-log 4-parameter variable slope model. Data for each variant was normalized to the average percent infected of its respective viral control. Data points represent mean ± SEM for N=3 replicates. C.) IC50 values for niclosamide potency against SARS-CoV-2 variants of concern. Values were extracted from curve fitting using GraphPad 9.0 and include SEM error bars (WA1: 1664 ± 149 nM, B.1.1.7: 298 ± 23 nM, B.1.351: 440 ± 21 nM, B.1.617.2: 774 ± 58 nM, P.1: 399 ± 34 nM). Significance was determined using Student’s T-tests (* = P<0.05, ** = P<0.01).
Furthermore, the current structure-activity examination uncovered certain mechanistic aspects of niclosamide. Most importantly, following the removal of the hydroxyl group of protonophore, both cell models employed in this study stopped functioning. This inference indicated that nonspecific endolysosomal neutralization was the major MOA.
Analogs' potency was dependent heavily on how weakly acidic and lipophilic they were. Since carboxylic acid substituents were present, analogs with high predicted acidity (pKa) were typically entirely inactive. The team also discovered that other protonophores, such as oxyclozanide and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), demonstrated anti-SARS-CoV-2 activity. This observation indicated that niclosamide's capacity to interfere with pH gradients was essential to its MOA.
Conclusions
Overall, the current study illustrated that niclosamide was not a good choice for COVID-19 treatment, considering its limited bioavailability, nonspecific protonophore function, variant-dependent efficacy, weak selectivity index, and complex polypharmacology. Nevertheless, the findings demonstrated that niclosamide's aniline and salicyl rings could be modified to alter selectivity and bioavailability while maintaining the drug's action. Thus, niclosamide provides a helpful chemical probe that could be exploited in a massive structure-activity relationship (SAR) effort to build better analogs in the future.
The researchers pointed out that longer niclosamide exposures at effective anti-SARS-CoV-2 concentrations probably result in noticeable side effects, which restricts practical use. Hence, the safety of niclosamide at antiviral titers in vivo needs further investigation.
Besides, de novo medication development for COVID-19 may benefit from additional research into the molecular variations behind the SARS-CoV-2 variant-based responses to medicines like niclosamide.
Moreover, even though the current work reveals that niclosamide and similar mitochondrial uncouplers have anti-SARS-CoV-2 potency, additional research was necessary to determine whether these MOA are linked to protonophore activity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment; Jesse W. Wotring, Sean M. McCarty, Khadija Shafiq, Charles J. Zhang, Theophilus Nguyen, Sophia R. Meyer, Reid Fursmidt, Carmen Mirabelli, Martin C. Clasby, Christiane E. Wobus, Matthew J. O'Meara, Jonathan Z. Sexton, bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.24.497526, https://www.biorxiv.org/content/10.1101/2022.06.24.497526v1
- Peer reviewed and published scientific report.
Wotring, Jesse W., Sean M. McCarty, Khadija Shafiq, Charles J. Zhang, Theophilus Nguyen, Sophia R. Meyer, Reid Fursmidt, et al. 2022. “In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment.” Vaccines 10 (8): 1284. https://doi.org/10.3390/vaccines10081284. https://www.mdpi.com/2076-393X/10/8/1284.