The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the coronavirus disease 2019 (COVID-19). Since its emergence at the end of 2019, SARS-CoV-2 has infected over 550 million and caused over 6.3 million deaths globally.
The rapid spread of SARS-CoV-2 significantly impacted national economies, while also causing severe disruptions to both national and international travel. In the absence of effective therapeutics, monoclonal antibodies (mAbs) were developed to counter the severity of symptoms and disease progression.
Study: Effect of Casirivimab/Imdevimab Treatment on Serum Type I Interferon Levels in SARS-CoV-2 Infection. Image Credit: Lightspring / Shutterstock.com
A new Viruses study determines the effect of casirivimab/imdevimab (C/I), which is a commonly used mAb combination therapy, on type 1 interferon (IFN-1) and cytokines during SARS-CoV-2 infection.
Introduction
Neutralizing mAbs are recombinant antibodies derived from the B lymphocytes of patients who have recovered from infection or from humanized mice.
Therapeutic mAbs provide passive immunity by directly neutralizing the virus and thus prevent its binding and entry into the target host cell. Furthermore, mAbs may label the infected cells and virus particles for phagocytosis or induce antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, and/or complement-dependent cytotoxicity.
Antibody administration may also cause increased disease severity by boosting the levels of inflammatory and infection-linked molecules, such as cytokines and chemokines, thereby activating immune cells and the complement cascade. Moreover, the attachment of the antibody to the cognate receptors on immune cells may drive the virus particles to bind to other antigen-receptor components of the antibody, thus infecting these cells at a higher rate. Taken together, these effects are referred to as “antibody-dependent enhancement (ADE)” of disease.
The C/I cocktail consists of two immunoglobulin G1 (IgG1) molecules that bind to two distinct non-overlapping epitopes of the viral spike protein’s receptor binding domain (RBD). To date, C/I not been reported to cause significant ADE and instead has been shown to reduce both hospitalization and mortality rates among patients with mild to moderate COVID-19, especially those who are high risk for severe disease.
The present study examined how C/I affected the immune response in early disease. IFN-1, which includes both IFN-α and IFN-β, plays a notable role in the initial immune response to SARS-CoV-2, while interleukin-6 (IL-6) and CXCL10 are both pro-inflammatory cytokines that may be responsible for the hyperactive immune response known as cytokine storm that can occur in severe and critical COVID-19. These biomolecules were therefore selected as biomarkers of interest in the current study.
Study findings
While the majority of patients who received early C/I treatment had worsened fever, a third of these individuals developed hypoxemia after the administration of the mAb cocktail. However, clinical symptoms resolved rapidly within two to three days, subsequently reducing the hospitalization duration to only five days in this subset of patients. Notably, patients who received a combination of remdesivir and dexamethasone (the R/D group) had an average hospital stay of about 10 days.
Even in hypoxemic patients, the need for supplemental oxygen was shorter at about two days as compared to nine days for those in the R/D group. White cell counts were also lower in patients receiving C/I, with fewer neutrophil levels as compared to lymphocyte levels. IFN-1 levels, particularly IFN-α, were also reduced to about 1% of their baseline values following R/D treatment, whereas IFN-β levels were reduced by about 75%.
This reduction in IFN-1 levels was similarly observed after C/I treatment; however, the magnitude of these improvements were only comparable for IFN-α, rather than IFN-β. In fact, IFN-β levels remained higher in the C/I group than in the R/D group; however, CXCL10 levels significantly declined following R/D treatment.
In the late immune response, this improvement was greater in terms of fever and hypoxemia, as well as IFN-1 and CXCL10 levels, in the C/I group. All three cytokines declined after treatment to levels similarly observed in the early phase of infection.
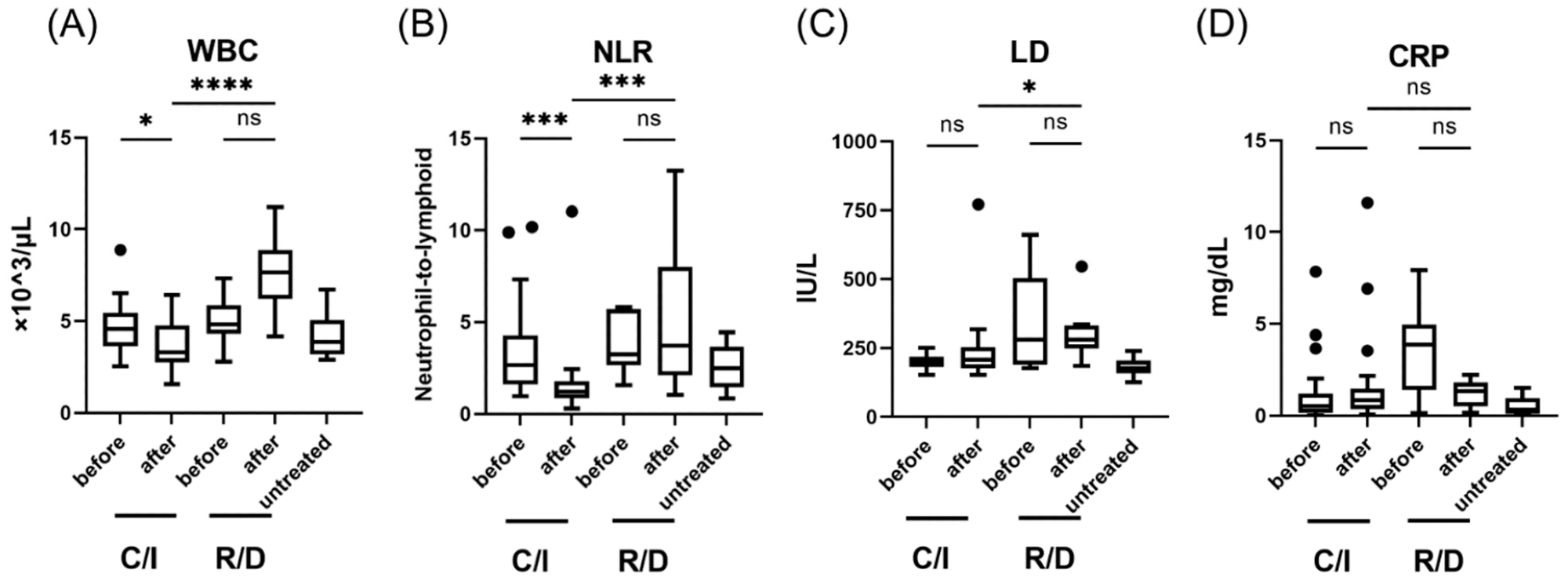 Laboratory findings in patients with SARS-CoV-2 infection, treated in the early phase (within 5 days after the onset of symptom); WBC (A), NLR (B), LD levels (C), and CRP levels (D). Each level was evaluated at the time point before and after the treatment; within 24 h before the initiation of therapy, 3 days after C/I administration or 5 days after R/D initiation. Data are presented as Tukey boxplots and individual values. Non-parametric Mann–Whitney test with Bonferroni correction was used to compare values between groups. *, p < 0.05. ***, p < 0.001. ****, p < 0.0001. C/I, casirivimab/imdevimab group; NLR, neutrophil-to-lymphoid ratio; ns, not significant; R/D, remdesivir/dexamethasone group; WBC, white blood cell count.
Laboratory findings in patients with SARS-CoV-2 infection, treated in the early phase (within 5 days after the onset of symptom); WBC (A), NLR (B), LD levels (C), and CRP levels (D). Each level was evaluated at the time point before and after the treatment; within 24 h before the initiation of therapy, 3 days after C/I administration or 5 days after R/D initiation. Data are presented as Tukey boxplots and individual values. Non-parametric Mann–Whitney test with Bonferroni correction was used to compare values between groups. *, p < 0.05. ***, p < 0.001. ****, p < 0.0001. C/I, casirivimab/imdevimab group; NLR, neutrophil-to-lymphoid ratio; ns, not significant; R/D, remdesivir/dexamethasone group; WBC, white blood cell count.
Implications
The researchers have demonstrated the ADE effect of the C/I cocktail in terms of a short-lived worsening of fever and the development of hypoxemia in about 60% and 40% of treated patients, respectively, which corroborates earlier reports. However, it appears that this effect is associated with the SARS-CoV-2 Delta variant, rather than other SARS-CoV-2 variants; thus, these effects could be referred to as an ADE-like response, rather than a true ADE response.
The rapid pace of recovery of the C/I cohort indicates the possibility that the reduction in IFN-1 might be favorable in this infection, even though it is a critical antiviral molecule and suppressor of viral replication in infected cells. This positive effect might be mediated by the neutralization of SARS-CoV-2 and the overall immune response by antibodies.
Since C/I may neutralize SARS-CoV-2 particles in the blood, rather than in the nasopharynx and other organs, this may lead to a fall in viral load in the blood and, as a result, declining IFN-1 levels. This is primarily observed with IFN-α, as this molecule is produced by hemopoietic cells in the blood, rather than IFN-β, which is more likely to be released by infected cells.
The C/I cocktail may also activate antigen-dependent immunity to SARS-CoV-2, thereby shifting from the innate (IFN-1) response to the adaptive arm. This corresponds with previous findings that an earlier adaptive response to the virus is associated with mild COVID-19, which underlines the importance of this effect of C/I.
This is the first study to demonstrate the therapeutic effect of C/I on the innate immune response.”
The study findings suggest that IFN-1, which was described as having a positive outcome when given early in COVID-19, might be less effective if combined with C/I. Secondly, the IL-6 blocker tocilizumab might reduce the ADE-like response to C/I, making this a potentially useful combination.
These findings will encourage further research on the effects of mAbs on innate and adaptive immune responses, which is crucial for the establishment of a novel therapeutic approach.”
Journal reference:
- Nagaoka, K., Kawasuji, H., Takegoshi, Y., et al. (2022). Effect of Casirivimab/Imdevimab Treatment on Serum Type I Interferon Levels in SARS-CoV-2 Infection. Viruses. doi:10.3390/v14071399.