Various studies have reported the impact of medium and severe coronavirus disease 2019 (COVID-19) cases on hospitalizations and related mortalities. However, more research is required to understand the effect of mild COVID-19 on antibody responses more than six months following the infection.
 Study: PERSISTENT IMMUNITY AFTER MILD SARS CoV-2 INFECTION - THE CoNAN-LONG TERM STUDY. Image Credit: DariaRen / Shutterstock
Study: PERSISTENT IMMUNITY AFTER MILD SARS CoV-2 INFECTION - THE CoNAN-LONG TERM STUDY. Image Credit: DariaRen / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers assessed the long-term immunity in persons having mild COVID-19 infection up to one year after the infection.
A total of 626 individuals were included in the first study round held in April 2020. Among these, 162 persons with a history of SARS-CoV-2 infection along with control participants who were matched based on gender and age were invited to participate in the second and third rounds of the study. A total of 146 participants were a part of the second visit held in October 2020, while 224 participated in the third visit held in April 2021. The team determined the antibody levels of all the participants. Furthermore, T cell analysis was conducted for all individuals having a history of infection. For all three study visits, the team conducted matched analysis of antibody levels of 40 participants.
At the 1.5-month time point or in the CoNAN 1 study, a total of 56 individuals were found to be anti-SARS-CoV-2 antibody seropositive (AB+), among which 44 and 46 participated in the second and the third rounds, respectively. Another 40 participants who were also tested for the antibody course were deemed as the ‘infected group.’
Furthermore, the team estimated the serum-antibody concentrations observed more than a year after SARS-CoV-2 infection. This was achieved by performing tests such as electronic data interchange (EDI) and Roche, which identified anti-nucleocapsid antibodies, Liason and Euroimmune, which identified anti-spike antibodies, and Maglumi Snibe, which identified anti-spike as well as anti-nucleocapsid antibodies.
The team assessed SARS-CoV-2 T cell-mediated immunity by analyzing spike protein-specific CD154+ 4-1BB+ cells found in TH cells. This immunity was determined by matching a cohort of 46 participants of the first and third visits, including 16 control persons and 30 infected patients. Furthermore, the spike-reactive TH cells were identified by comparing TH cells that were restimulated by covering either the N-terminal or the C-terminal part of the SARS-CoV-2 spike protein to TH cells that responded only in the presence of dimethyl sulfoxide (DMSO).
Results
The study results showed that 161 participants of the third visit of the CoNAN 1 study were AB- among which 40 became AB+. Furthermore, nine of the participants tested PCR-positive for COVID-19 between the second and the third visit. Moreover, 21 participants did not receive their vaccination. The participants from the infected group had a median age of 60.5 years, including 57.43% of men and 42.86% of female participants who tested positive for antibodies in the first visit and reported neither COVID-19 vaccination nor reinfection.
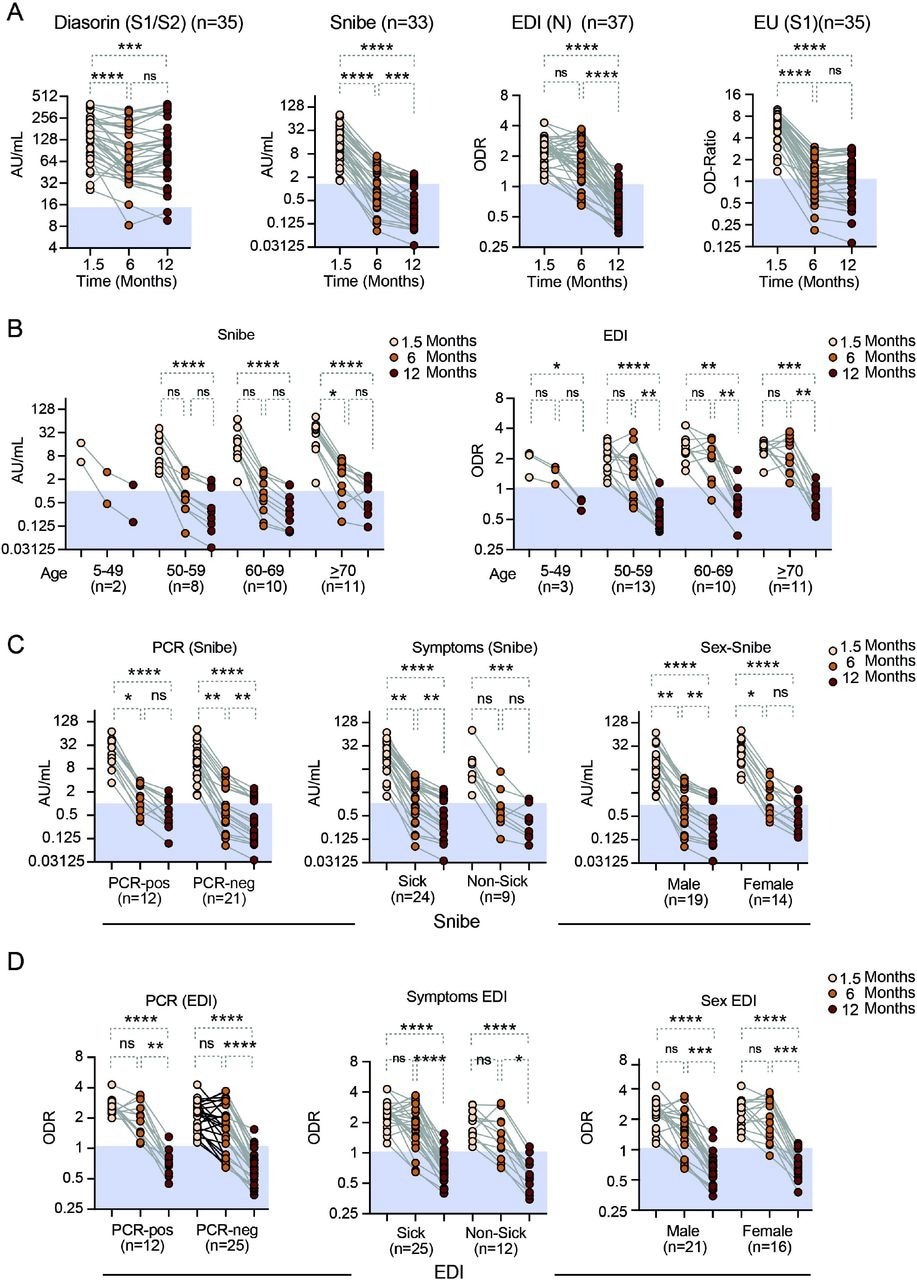
Anti-SARS-CoV-2 antibody levels over time as assessed with the three quantitative and one semiquantitative (EU) antibody tests as indicated A) all participants. Stratified by B) age for the Snibe (left panel) and EDI test (right panel). C) Results from the Diasorin Snibe antibody test stratified for PCR status (left panel), asymptomatic vs. symptomatic disease (middle panel) and sex (right panel). D) same as C for EDI test. N= number of individuals per group. Friedman test with Dunns post-hoc analysis. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. Abbreviations: AU.. arbitrary units, ns.. non-significant (p>0.05), Snibe: SN.. 2019-nCoV IgG kit (Snibe Co., Ltd., Shenzhen, China); ED.. EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics Inc., San Diego, USA).
Quantitative tests like EDI, Liaison, Maglumi Snibe, and Euroimmune showed that over the observation period of one year, there was a substantial reduction in the serum antibody concentrations. This decline in antibody concentrations varied among different time points and individual tests. A comparison of the reduced antibody concentrations between the EDI and the Euromimmune tests in the duration between 1.5 and six months revealed that the decline in the number of anti-nucleocapsid antibodies was less significant. This effect was found consistent even after 12 months.
However, after 12 months, the Diasorin assay showed a less remarkable reduction in antibody concentrations, indicating an effect that was test-specific and not antigen-specific. This also suggested a rapid waning in the concentration of serum antibodies which were detected in some of the tests in the first six months. Furthermore, a less pronounced waning and preservation of antibodies were observed within one year after the COVID-19 diagnosis.
The team found the presence of SARS-CoV-2 spike-specific CD154+ 4-1BB+ TH cells at 1.5 and 12 months after COVID-19 diagnosis. Moreover, there was a slight but notable decline in spike-specific TH cells among individuals with a history of COVID-19 infection over time. Only 6.7% of the patients reported the total disappearance of spike-specific TH cell response after a year of primary infection. Furthermore, in comparison to the SARS-CoV-2-negative individuals, participants who had a previous history of SARS-CoV-2 infection revealed a higher proportion of SARS-CoV-2 specific T cells at different time points tested.
Overall, the study findings showed the persistence and robustness of TH cell immunity elicited after a mild SARS-CoV-2 infection. While the antibody concentrations declined to a detection minimum after a year of infection, the specific TH cell responses were still detectable.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
PERSISTENT IMMUNITY AFTER MILD SARS CoV-2 INFECTION - THE CoNAN-LONG TERM STUDY - Clara Schnizer, Nico Andreas, Wolfgang Vivas Varela, Thomas Kamradt, Michael Baier, Michael Kiehntopf, Stefan Gloeckner, Andre Scherag, Bettina Loeffler, Steffi Kolanos, Joel Guerra, Mathias Pletz, Sebastian Weis, medRxiv 2022.07.05.22277237, DOI: https://doi.org/10.1101/2022.07.05.22277237, https://www.medrxiv.org/content/10.1101/2022.07.05.22277237v1
- Peer reviewed and published scientific report.
Schnizer, Clara, et al. "Persistent Humoral and CD4+ TH Cell Immunity after Mild SARS-COV-2 Infection—The CoNAN Long-Term Study." Frontiers in Immunology, vol. 13, 2023, https://doi.org/10.3389/fimmu.2022.1095129, https://www.frontiersin.org/articles/10.3389/fimmu.2022.1095129/full