Due to a lack of longitudinal tissue samples, the mechanistic basis of post-acute sequelae of SARS-CoV-2 (PASC)-related lung abnormalities is barely understood. Moreover, little is known about the underlying mechanisms governing non-viral chronic active pneumonia (CAP) or pulmonary fibrosis (PF) in humans, providing incomplete roadmaps for studies of SARS-CoV-2 pulmonary pathogenesis. Here it is also noteworthy that human autopsy samples are heterogeneous and describe disease only at a specific time. Therefore, they do not elucidate the pathogenesis of post-SARS-CoV-2 lung disease.
Mice infected with MA10 suffer from an acute respiratory distress syndrome (ARDS) similar to humans. Therefore, BALB/c mice present an opportunity to investigate PASC pathogenesis from acute to clinical recovery phases. Additionally, this murine model facilitates testing countermeasures to ameliorate PASC. Previous studies have also not described PASC-like disease phenotypes in the lung after virus clearance.
About the study
In the present study, researchers inoculated 103 plaque-forming units (PFU) of SARS-CoV-2 MA10 in one-year-old female BALB/c mice to induce severe acute disease. Likewise, they inoculated 10-week-old mice with a higher inoculum of MA10 (104 PFU) to induce similar disease severity.
As per the recommendations for diagnosing different COVID-19 phases in humans, the team necropsied these mice at two, seven, 15, 30, 60, and 120 days post-infection (dpi). They used the retrieved samples for the study analyses.
The team used complementing virological and histological methods to assess lung damage in surviving mice. Further, they utilized digital spatial profiling (DSP) to identify transcriptional profiles during acute and chronic disease phases to characterize tissue damage and repair in mice and humans. The team supplemented these techniques with immunohistochemistry (IHC) and computed tomography (CT) scanning. They also used ribonucleic acid in situ hybridization (RNA-ISH) to validate data obtained from DSP analyses. Lastly, they investigated measures to identify early biomarkers to identify PASC and evaluated countermeasures to prevent lung disease during PASC.
Study findings
The older mice that survived MA10 infection cleared the infection by 15 dpi. Like humans, they had damaged pulmonary epithelia that turned into persistent pulmonary lesions, and micro-CT also revealed subpleural opacities and fibrosis.
The lesions were heterogeneous and varied in severity between 30 to 120 dpi. Further, these mice had abnormally repairing alveolar epithelial type II (AT2) cells and interstitial macrophages alongside persistent lung lesions. In subpleural regions, they exhibited myofibroblast proliferation, accumulated lymphoid cells, and deposited interstitial collagen.
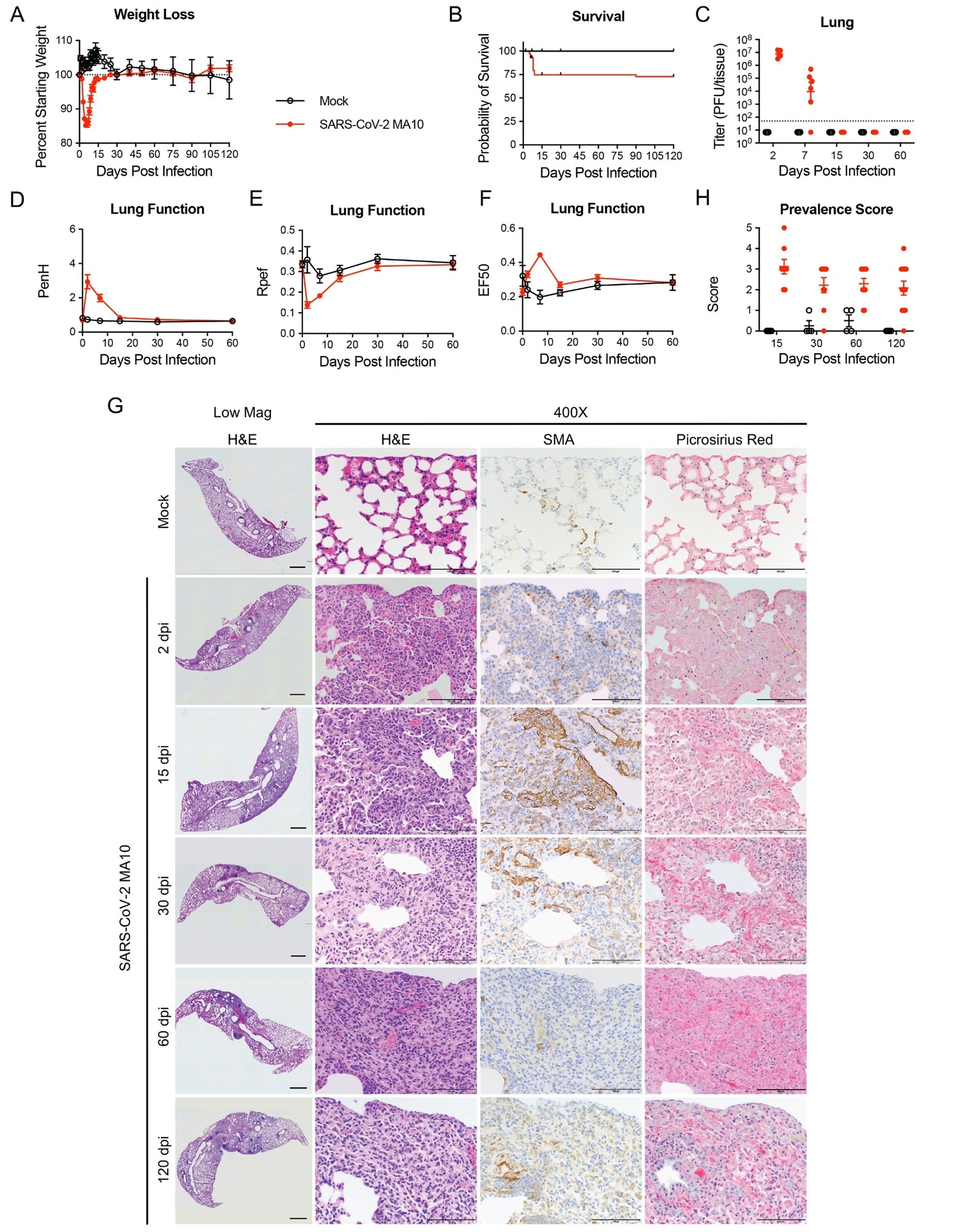 SARS-CoV-2 MA10 infection causes lung damage in aged surviving mice. 1-year-old female BALB/c mice were infected with 103 PFU SARS-CoV-2 MA10 (n=74) or PBS (n=24) and monitored for (A) percent starting weight and (B) survival. (C) Log transformed infectious virus lung titers were assayed at indicated time points. Dotted line represents LOD. Undetected samples are plotted at half the LOD. (D to F) Lung function was assessed by whole-body plethysmography for (D) PenH, (E) Rpef, and (F) EF50. (G) Histopathological analysis of lungs at indicated time points are shown. H&E indicates hematoxylin and eosin staining. SMA indicates DAB-labeling (brown) immunohistochemistry for α-smooth muscle actin. Picrosirius Red staining (bright pink-red) highlights collagen fibers. Image scale bars represents 1000 μm for low magnification and 100 μm for 400X images. (H) Disease incidence scoring is shown for indicated time points: 0 = 0% of total area of examined section, 1 = less than 5%; 2 = 6 to 10%; 3 = 11 to 50%; 4 = 51 to 95%; 5 = greater than 95%. Graphs represent individuals necropsied at each time point (C and H), with the average value for each treatment and error bars representing standard error of the mean. Mock infected animals represented by open black circles and SARS-CoV-2 MA10 infected animals are represented by closed red circles.
SARS-CoV-2 MA10 infection causes lung damage in aged surviving mice. 1-year-old female BALB/c mice were infected with 103 PFU SARS-CoV-2 MA10 (n=74) or PBS (n=24) and monitored for (A) percent starting weight and (B) survival. (C) Log transformed infectious virus lung titers were assayed at indicated time points. Dotted line represents LOD. Undetected samples are plotted at half the LOD. (D to F) Lung function was assessed by whole-body plethysmography for (D) PenH, (E) Rpef, and (F) EF50. (G) Histopathological analysis of lungs at indicated time points are shown. H&E indicates hematoxylin and eosin staining. SMA indicates DAB-labeling (brown) immunohistochemistry for α-smooth muscle actin. Picrosirius Red staining (bright pink-red) highlights collagen fibers. Image scale bars represents 1000 μm for low magnification and 100 μm for 400X images. (H) Disease incidence scoring is shown for indicated time points: 0 = 0% of total area of examined section, 1 = less than 5%; 2 = 6 to 10%; 3 = 11 to 50%; 4 = 51 to 95%; 5 = greater than 95%. Graphs represent individuals necropsied at each time point (C and H), with the average value for each treatment and error bars representing standard error of the mean. Mock infected animals represented by open black circles and SARS-CoV-2 MA10 infected animals are represented by closed red circles.
These mice also had elevated levels of several pro-inflammatory and pro-fibrotic cytokines. These included interleukin-1Beta (IL-1β), IL-33, IL-17A, tumor necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor growth factor-beta (TGF-β).
Although most cytokines returned to their normal levels by 30 dpi, sub-pleural fibrotic regions exhibited prolonged up-regulation of TGF-β signaling, as observed during DSP and RNA-ISH. Previous studies have observed similar heterogeneous cellular and fibrotic features in subpleural regions of late-stage COVID-19 patients.
The infection in bronchioles, particularly in subpleural regions, provided cues to the etiology of the late-stage alveolar CAP/PF response. Despite similar infections, bronchioles were repaired without any evidence of fibrotic sequelae. In all probability, tissue-specific ISG responses protected bronchioles from this adverse fate.
Furthermore, CD4+ and CD8+ T cell populations increased in SARS-CoV-2-diseased areas of mouse lungs, and peripheral lymphoid aggregations characterized chronic disease. Consistent with human studies, DSP and flow cytometry data confirmed the expansion of the interstitial macrophage population. Most importantly, the study data confirmed that replication-defective and pro-inflammatory transitional cells, including alveolar differentiation intermediate (ADI)/ damage-associated transient progenitor (DATP)/pre-AT1 transitional cell state (PATS) emerges early after SARS-CoV-2 infection and persists with continued inflammation and failure of repair.
The authors first observed these cells at two dpi in the test animals, and they persisted through 30 dpi in diseased but not morphologically intact alveolar regions. Histological studies evidenced the activation of ADI/DATP/PATS cells-related extracellular matrix pathways in the subpleural areas.
Early molnupiravir treatment weakened chronic PASC in the SARS-CoV-2 MA10 mouse model. Similarly, early administration of direct-acting antiviral, Nintedanib also blunted maximal fibrotic responses to SARS-CoV-2 between seven and 15 dpi. However, additional studies could confirm these findings and evaluate other anti-fibrotic drugs for PASC treatments.
Conclusions
Overall, the current study modeled chronic SARS-CoV-2 to help longitudinally study the molecular pathways mediating long-term COVID-19 pulmonary sequelae to evaluate treatments for human PASC. The study findings also provided cues to the role of host genetics in defining PASC outcomes. Regarding countermeasures, the SARS-CoV-2 MA10 model could help rapidly test agents that may counter the pulmonary CAP/PF effects of COVID-19 during longer clinical trials.
Journal reference:
- Kenneth H. Dinnon Iii, Sarah R. Leist, Kenichi Okuda, Hong Dang, Ethan J. Fritch, Kendra L. Gully, Gabriela De La Cruz, Mia D. Evangelista, Takanori Asakura, Rodney C. Gilmore, Padraig Hawkins, Satoko Nakano, Ande West, Alexandra Schäfer, Lisa E. Gralinski, Jamie L. Everman, Satria P. Sajuthi, Mark R. Zweigart, Stephanie Dong, Jennifer Mcbride, Michelle R. Cooley, Jesse B. Hines, Miriya K. Lovesteve D. Groshong, Alison Vanschoiack, Stefan J. Phelan, Tyler Hether, Michael Leon, Ross E. Zumwalt, Lisa M. Barton, Eric J. Duval, Sanjay Mukhopadhyay, Edana Stroberg, Alain Borczuk, Leigh B. Thorne, Muthu K. Sakthivel, Yueh Z. Lee, James S. Hagood, Jason R. Mock, Max A. Seibold, Wanda K. O’neal, Stephanie A. Montgomery, Richard C. Boucher, Ralph S. Baric, SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice, Science Translational Medicine 2022, DOI: 10.1126/scitranslmed.abo5070, https://www.science.org/doi/10.1126/scitranslmed.abo5070