I am Morteza Sarmadi, a doctoral research specialist at MIT Langer Lab supervised by Prof. Robert Langer and Dr. Ana Jaklenec. I completed my Ph.D. in Mechanical Engineering in the Jaklenec group at the MIT Langer Lab. I work on designing and developing micro/nanoscale devices for biomedical applications. I am particularly passionate about improved drug delivery for vaccination and cancer therapy.
What inspires me about this topic is its substantial impact on global health, which can save human lives. It is estimated that each year 10 million infants do not receive proper immunity against viruses due to difficulties in the current vaccination schedule which requires multiple injections. Our work (Jaklenec group) at MIT focuses on one day helping to solve this issue and potentially saving millions of lives worldwide by improving vaccination coverage.
Image Credit: WESTOCK PRODUCTIONS/Shutterstock.com
Multiple doses of a vaccine often need to be administered to be considered fully vaccinated, as seen with COVID-19. Why is this?
Briefly, it goes back to the biology of how our body generates immunity against viruses for a given vaccine. Having two or multiple shots delivered to the body months apart helps the body develop a memory so a sufficient number of antibodies against the virus can be generated upon exposure.
It's more of a safety check that ensures our body is fully prepared to fight against viral infection. It also goes back to the molecular structure of the vaccine and how it is supposed to activate our immune system against viruses.
Administering multiple vaccine doses to a single patient can be challenging, time-consuming, and often costly. How does your new technique help to overcome these challenges?
Our group at MIT has developed a new method for administering multiple vaccine doses based on time-release microparticles. These microparticles can maintain and protect the vaccine doses inside the body and release them in the body at predetermined time points. The time point for the release can be programmed to mimic the same schedule patients need to get booster shots.
Therefore, by injecting a cocktail of these microparticles, each releasing the vaccine at the right dose and time, we can deliver all shots with the first injection and eliminate the need for injecting vaccine doses multiple times. These microparticles are made from safe materials completely compatible with the human body and automatically dissolve over time in the body.
We believe this technique can significantly reduce the need to visit a healthcare provider to receive booster shots, a major challenge in remote areas without sophisticated healthcare resources. It could also be a lot more convenient if we had to receive only a single shot of COVID-19 vaccines (in the case of Moderna & Pfizer vaccines) and could help reach herd immunity faster in future pandemics.
In your latest research, you have developed microparticles that could be used to create "self-boosting vaccines". Can you tell us more about how you developed these particles and their potential use within vaccinations?
In 2017, we introduced a new manufacturing process for fabrication of a new class of microparticles termed "core-shell" microparticles that are compatible even with sensitive cargo such as vaccines. This work was published in the Journal Science and focused on how to fabricate an extensive array of these particles.
The latest work shows how these particles work and how they can be used to deliver multiple doses of drugs or vaccines with a single injection.
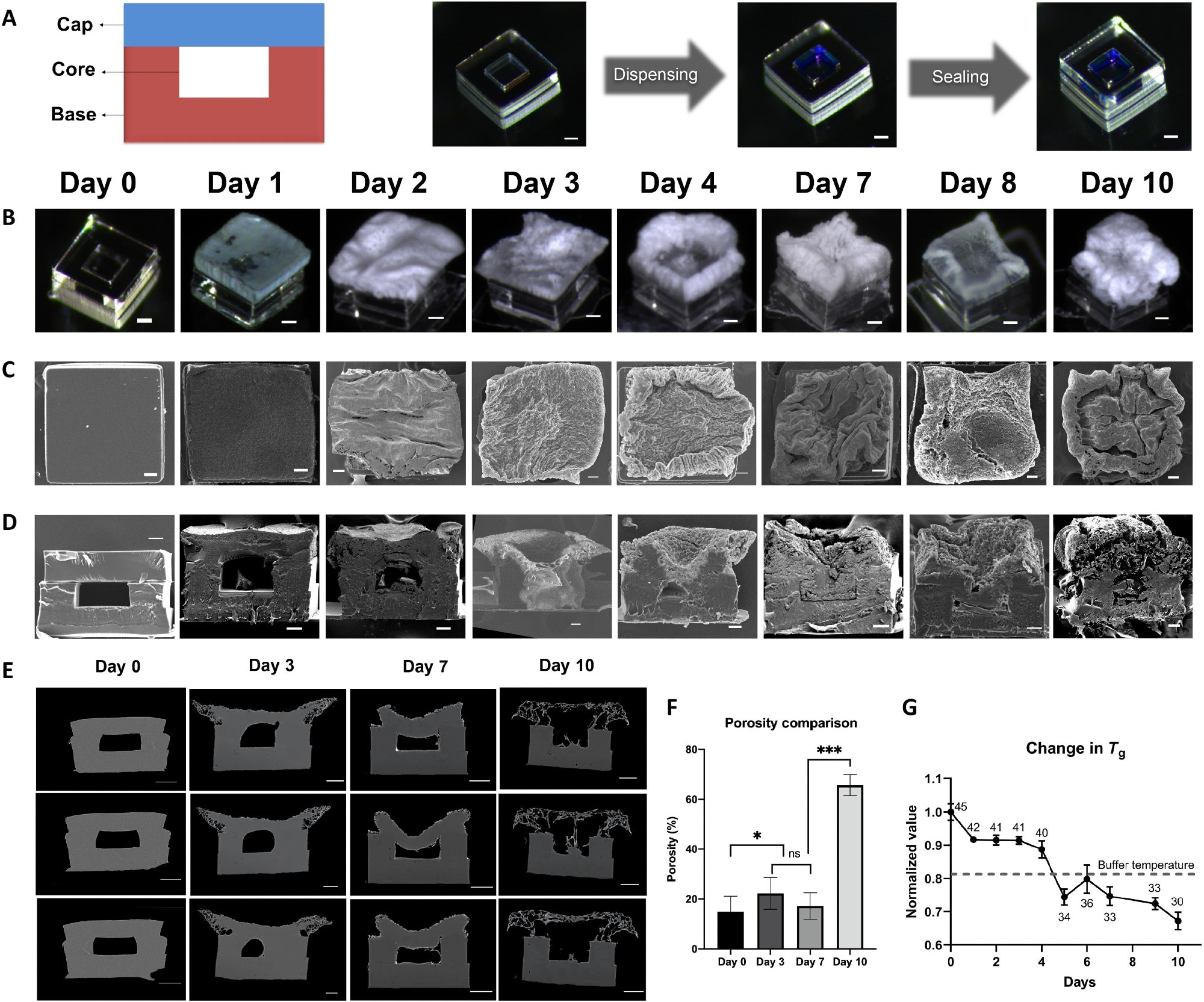
Image Credit: Morteza Sarmadi, Massachusetts Institute of Technology.
With a potential "self-boosting" vaccine, there could be an issue regarding their stability over time. How does your latest research also investigate this?
An important feature of the self-boosting vaccine doses delivered by microparticles is that they are protected against degradation in the body. As an additional step in the latest work, we also studied how to reduce the acidity of the particles once injected into the body to further protect the stability of the vaccines over time.
Moreover, this technology enables vaccines to be maintained in solid forms and we have found a number of stabilizing excipient combinations that can be used for this purpose. This is an additional feature that can substantially help in stabilizing particle-delivered vaccines compared to soluble vaccines.
Combined, we believe that, unlike traditional vaccine delivery techniques, self-boosting vaccine doses delivered by these particles would retain their ability to activate the immune system in the body successfully.
Despite its advantages in increasing patient compliance and healthcare resources, are there any other potential benefits these "self-boosting" vaccines could offer?
Traditional vaccines are in liquid form, hard to ship, and require low temperature for storage. Conversely, vaccines encapsulated in these self-boosting microparticles are dried and can be more easily shipped worldwide. When stored properly without exposure to humidity, these particles can improve vaccine shelf-life and maintain the encapsulated vaccine stable even at room temperature.
We believe this could potentially be a significant benefit to the global distribution of vaccines currently hampered by the cold chain issue.
Since the start of the COVID-19 pandemic, the public has been increasingly interested in virology, infectious diseases, and vaccines. How has this increased awareness helped your research? Why is it important that the public understand vaccines and their uses?
Since the start of COVID-19, we have significantly ramped up research on self-boosting vaccines. This awareness has positively impacted research in vaccinations not only in our group at MIT but also globally. It is essential that the public trust vaccines. A strong body of scientific studies supports the safety and efficacy of vaccines, especially in children.
Lack of vaccination not only can be risky for the individual's own well-being, but it can also threaten others' safety who come in close contact with the individual, therefore threatening the well-being of our society.

Image Credit: Pandagolik1/Shutterstock.com
What further research is needed to be carried out before these vaccines become frequent within healthcare?
These vaccines need to go through the regular FDA approval process, including clinical trials, to assess safety and efficacy in humans.
Do you believe these microparticles could potentially be used to deliver other therapeutics, including cancer drugs? What would this mean for patients who travel multiple times to a hospital?
These particles are a platform technology that can be used for other applications beyond self-boosting vaccines. Any applications requiring multiple injections on a regular basis can potentially benefit from this technology. Examples include cancer drugs, insulin, growth hormones, and autoimmune diseases.
What's next for you and your research?
We are currently using these particles to deliver self-boosting vaccine doses against some of the deadliest infectious diseases in the world. We have been evaluating the particles to make sure they are compatible with the vaccines and can provide sufficient immunity. Our goal is to translate this technology from lab to marketplace to ensure it can make a difference in patients' lives.
Where can readers find more information?
Jaklenec group website: https://jaklenecgroup.mit.edu/
Link to the article: https://www.science.org/doi/10.1126/sciadv.abn5315
Link to our original publication back in 2017: https://www.science.org/doi/full/10.1126/science.aaf7447
About Morteza Sarmadi
Morteza is currently a doctoral research specialist at MIT following completing his PhD there. He finished his PhD in Mechanical Engineering at the laboratory of Prof. Robert Langer at MIT. He worked within Dr. Ana Jaklenec's group in the Langer Lab on developing microscale polymeric-based biomaterials for the controlled delivery of vaccines. He has authored or co-authored more than 20 peer-reviewed publications, cited more than 300 times to date, and has two pending patents.
The clinical translation of biomedical technologies inspires his research with a significant impact on patients' lives and global healthcare. Before joining MIT, he completed his Masters' degree in Mechanical Engineering at the Sharif University of Technology, where he developed biomaterials for combined drug delivery and wound healing applications.