The continual emergence of novel SARS-CoV-2 variants has threatened the diagnostic capacity of existing techniques and vaccine efficacy. The clinical presentation of COVID-19 may not be adequate to discriminate SARS-CoV-2 infections from other pulmonary infections, warranting the need for highly sensitive, specific, and accurate diagnostic techniques for SARS-CoV-2 detection in nasal secretions, saliva, blood, semen, or feces.
 Study: Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing. Image Credit: NIAID
Study: Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing. Image Credit: NIAID
About the review
In the present review, researchers comprehensively discussed SARS-CoV-2 detection in body fluids based on the biomarkers used (i.e., surface antigens, antibodies, and nucleic acids) and techniques.
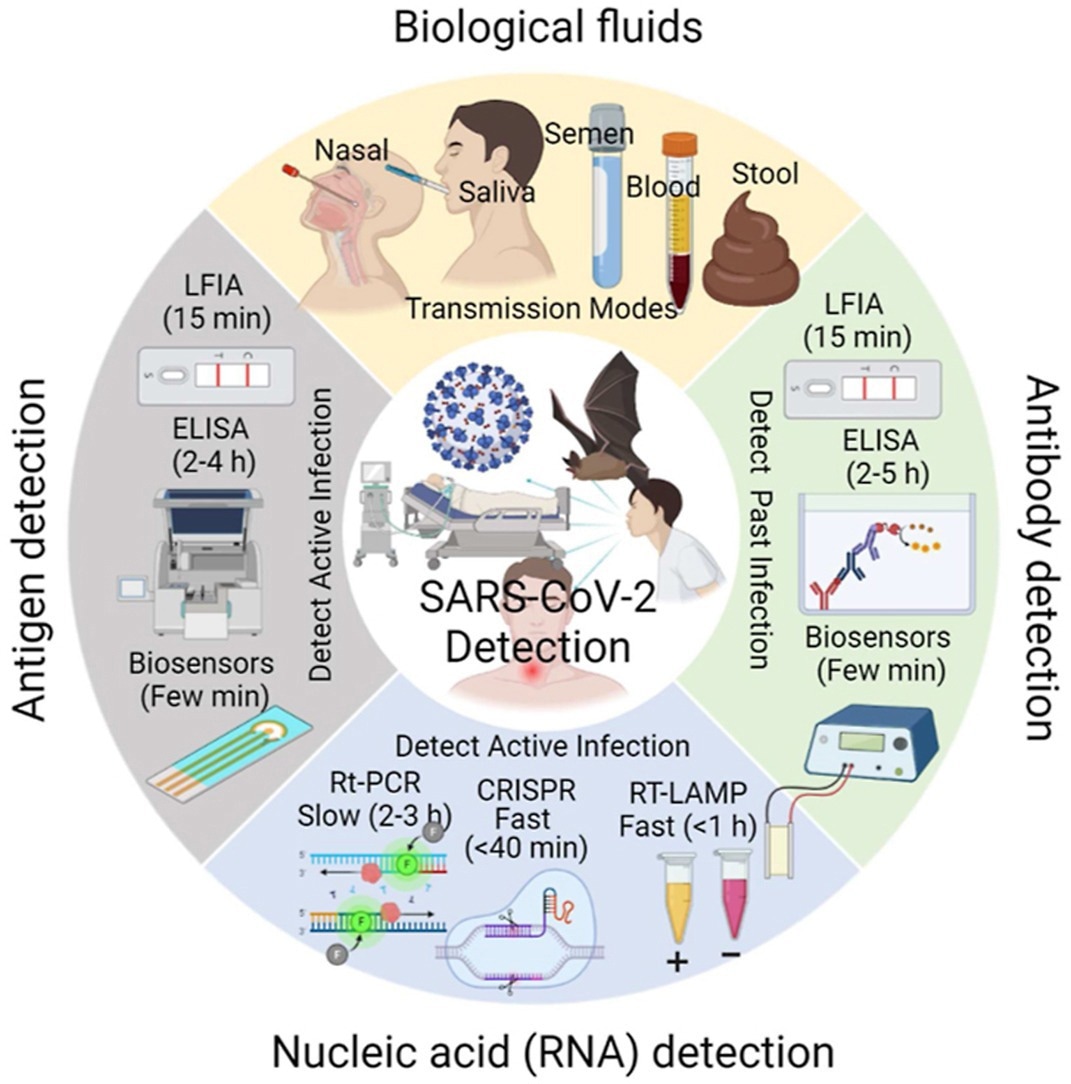
Most diagnostic techniques are based on SARS-CoV-2 antigens, ribonucleic acid (RNA), antibodies, and whole viruses. In addition, techniques such as lateral flow immunoassay (LFIA), enzyme-linked immunosorbent assay (ELISA), and biosensors are used to detect past infection based on anti-SARS-CoV-2 antibody presence or active infection based on SARS-CoV-2 antigen presence.
Molecular techniques for SARS-CoV-2 detection include reverse transcription-polymerase chain reaction (RT-PCR), RT loop-mediated isothermal amplification (RT-LAMP) and clustered regularly interspaced short palindromic repeats (CRISPR). While ELISA and RT-PCR require two to five ≥2 hours for SARS-CoV-2 detection, biosensors and LFIA can detect SARS-CoV-2 within a few minutes.
SARS-CoV-2 detection techniques can be broadly classified as (i) nucleic acid assays based on SARS-CoV-2 nucleic acid detection (e.g., RT-PCR, RT-LAMP, microarrays, CRISPR, RNA sequencing, and biosensors), and (ii) immunological assays, based on antigen or antibody detection (e.g., LFIA, ELISA, biosensors, and spectroscopy).
Nucleic acid assays for SARS-CoV-2 detection
Nucleic acid assays are used for direct, sensitive, and specific detection of SARS-CoV-2 RNA in the samples, the quantity of which denotes the presence or absence of COVID-19. RT-PCR is the gold standard for molecular detection of SARS-CoV-2 due to its high sensitivity and is based on nucleic acid amplification; however, RT-PCR may yield false-negative results due to viral RNA sequence variations, low viral loads, and inappropriate collection of upper respiratory tract samples. Further, RT-PCR is expensive, time-consuming, and requires trained personnel and equipment.
RT-LAMP assays are based on autocyclic deoxyribonucleic acid (DNA) strand displacement at room temperature and involve single-step reverse transcription and color change-based detection by the naked eye. The assays can detect SARS-CoV-2 RNA in oropharyngeal swabs, nasopharyngeal swabs, and serum samples with low viral loads (480 RNA copies). However, the assays are not performed for mass COVID-19 testing.
DNA microarray-based assays are used for rapid, accurate, sensitive, specific, and efficient analysis of gene expressions from nasopharyngeal swab samples and involve oligonucleotide and complementary DNA (cDNA) hybridization. CRISPR assays detect SARS-CoV-2 RNA using Cas13, which can excise reporter RNA sequences in response to activation by SARS-CoV-2-specific guide RNA. By CRISPR assays, SARS-CoV-2 can be detected easily using paper strips with high specificity or sensitivity. However, trained personnel and an additional DNA amplification step are required, making CRISPR assays less economical than RT-PCR and RT-LAMP.
Next-generation sequencing (NGS) is a capillary electrophoreses-based high-throughput technique for accurate and rapid SARS-CoV-2 sequencing. It is used to confirm RT-PCR and RT-LAMP results, especially for samples with low SARS-CoV-2 content. However, expensive equipment and chemical substances are required, limiting its use for point-of-care (POC) diagnosis.
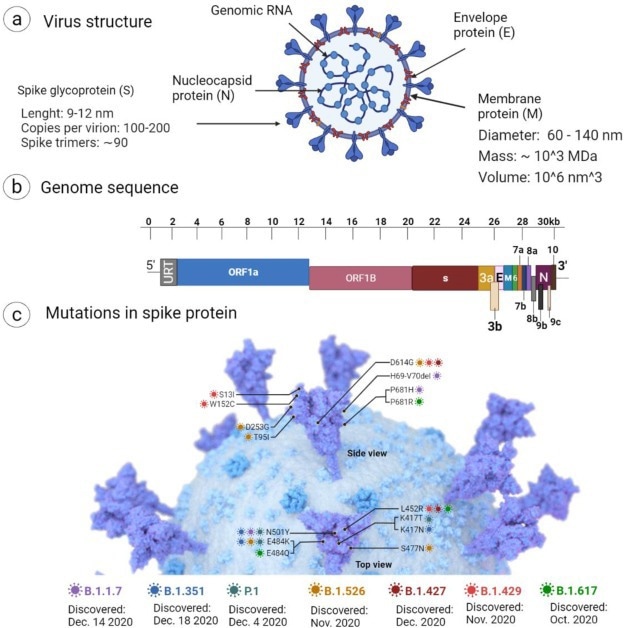
Schematic representation of (a) cartoon model showing the structure of SARS-CoV-2, (b) genome structure and encoded proteins, and (c) mutation in spike proteins. The image was created with Biorender.
Immunological assays for SARS-CoV-2 detection
Rapid antigen tests such as LFIAs are commonly used since they can be performed in the patient's vicinity for reliable and POC SARS-CoV-2 detection. Immunoglobulin M (IgM), IgA, and IgG antibodies are produced by the immune system against SARS-CoV-2, which can serve as indicators of infection. IgM production initially increases in the first phase of infection and then rapidly decreases, while IgG is produced in the second phase and remains in the blood after recovery.
LFIA involves IgG, IgM antibody binding to SARS-CoV-2 antigens and the formation of antigen-antibody complexes that bind to secondary antibodies (antihuman IgG and antihuman IgM antibodies) and form antibody sandwiches between SARS-CoV-2 nucleocapsid (N) protein and secondary antibodies. Negative and positive tests are indicated by one and two colored lines, respectively. LIFA has shown 103-fold and 105-fold lower sensitivity than virus culture and RT-PCR, respectively.
ELISA is a sensitive, high-throughput laboratory test that can detect both viral antigens and host antibodies to specific SARS-CoV-2 antigens such as N protein, spike (S) protein subunit 1 (S1), and S RBD. ELISA assays are highly sensitive and specific and used as additional diagnostic tools for RT-PCR.
Biosensors are analytical devices used for non-invasive, quantitative, rapid, extremely sensitive, and cost-effective POC SARS-CoV-2 detection based on the realization of binding of viral components (enzyme, antibody, peptide, or nucleic acid) to their receptors, detected by a transducer. Several types of biosensors are available such as optical, physical, electronic, and electrochemical biosensors. In addition, rapid, sensitive, and specific COVID-19 detection has also been reported by spectroscopic techniques such as Raman spectroscopy, ATR-FTIR (attenuated total reflectance-Fourier transform infrared) spectroscopy, and ultraviolet-visible (UV-vis) spectroscopy.
Overall, the review findings highlighted the diagnostic realm of COVID-19, including nucleic acid-based and immunoassay-based SARS-CoV-2 detection in body fluids.
Journal reference:
- M. Mostafa, A. Barhoum, E. Sehit, H. Gewaid, E. Mostafa, M.M. Omran, M.S. Abdalla, F.M. Abdel-Haleem, Z. Altintas, R.J. Forster, Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing, Trends in Analytical Chemistry (2022), doi: https://doi.org/10.1016/j.trac.2022.116750. https://www.sciencedirect.com/science/article/pii/S0165993622002333?via%3Dihub