In the current study, researchers hypothesized that nCOV-S(JET) would mount a detectable neutralizing antibody response when delivered via needle-free jet injection and pursued evidence of its immunogenicity in rhesus macaques, a non-human primate (NHP) model.
They vaccinated the test animals using two needle-free delivery methods. The first method used was the Stratis device to intramuscularly (IM) deliver 2 mg per vaccination dosage. The second attempted intradermal (ID) delivery of 0.4 mg per vaccination with the Tropis device. The Stratis and Tropis devices delivered vaccines as a jet of liquid IM or ID, respectively. The team measured vaccine-elicited neutralizing antibodies using two assays - i) live-virus plaque reduction neutralization tests (PRNT); ii) pseudovirion neutralization assays (PsVNA). Additionally, they performed a MAGPIX multiplex immunoassay, which utilizes the SARS-CoV-2 S, S1 subunit, receptor-binding domain (RBD), and nucleocapsid (NP) proteins.
The study used 12 Chinese-origin rhesus macaques aged eight to 15 years and weighing between five and 16 kilograms. Each vaccination group comprised randomly assigned three male and three female animals. The team vaccinated all the test animals on days zero, 21, and 42 and collected their whole blood samples on days zero, 21, 35, 63, and 168. They monitored all test animals for clinical and behavioral anomalies daily.
Study findings
Rhesus macaques needed an additional (second) boost to reach similar neutralizing antibody titers as Syrian hamsters. The geometric mean titer (GMT) or PsVNA50 in hamsters was approximately 640 after two vaccinations, whereas it was 58 and 326 in rhesus macaques after two and three vaccinations, respectively. Likewise, GMT PRNT50 in hamsters after two vaccinations was approximately 640, whereas it was 24 and 71 in rhesus macaques after two and three vaccinations, respectively.
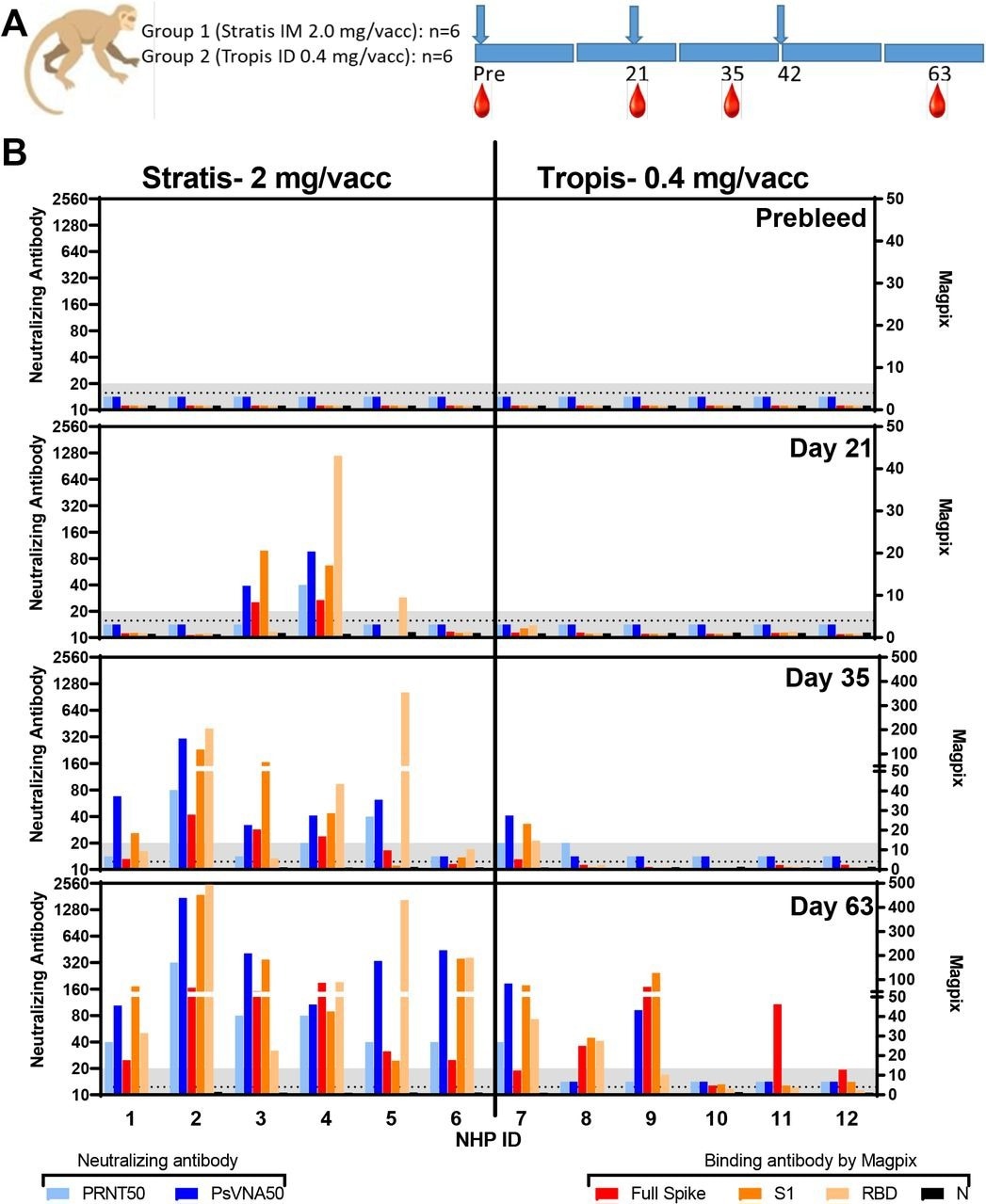
Neutralizing and binding antibody responses. PRNT50, PsVNA50, and Magpix titers from sera collected at various timepoints. A) Design. (blue arrows = vaccine dosing; red drops = blood collection time points). B) Neutralizing and binding antibody values at the indicated timepoints. Assay lower limits are shown as gray shaded area.
It is noteworthy that DNA vaccines exhibit the highest immunogenicity when administered IM compared to other routes. While hamsters received a total of 0.4 mg nCOV-S(JET) intramuscularly over three vaccinations, NHPs received a six mg dosage, which implies the NHPs received an inadequate dose compared to the hamsters on a per weight basis.
Nevertheless, this DNA vaccine delivered by needle injection protected NHPs from disease. It elicited neutralizing antibody titers over 100, as measured by a PsVNA. Another study tested a similar S-based DNA vaccine termed ZyCoV-D in rabbits. Three doses of its ID delivery using the Tropis device elicited neutralizing antibody titer of 108, as assessed via a microneutralization test. Thus, the neutralizing antibody titers elicited in NHPs appear comparable to titers that were protective in rabbits.
Furthermore, the nCOV-S(JET) vaccine delivered using the IM Stratis device exhibited cross-neutralizing activity against SARS-CoV-2 variants of concern (VOC), as per the PsVNA assessment. All the NHPs had a minimum PsVNA50 titer of 80 against SARS-CoV-2 WA-1 strain, Beta, and Delta VOCs. Notably, the neutralizing antibody titers against the Delta VOC were the highest.
On the contrary, DNA vaccine delivered ID by Tropis device had lower cross-neutralizing VOC responses. Only two animals (#7 and #9) showed cross-neutralizing antibodies against all VOCs, as measured by PsVNA, and only #7 showed detectable cross-neutralizing antibodies against all VOCs tested by PRNT. Animals #7 and #9 also had the most robust antibody binding response, as measured by the Magpix. Among its other benefits, the nCOV-S(JET) DNA vaccine was not formulated with lipid nanoparticles (LNPs) and did not require any adjuvant or electroporation. It just used relatively inexpensive disposable needleless syringes.
Conclusions
Future studies should explore ways to increase the potency of the nCOV-S(JET) DNA vaccine to enable its use as a standalone vaccine. However, for dosages used in the current study, it generated the desired neutralizing antibody responses following a two-dose regimen, which makes this vaccine most beneficial for heterologous boosting strategies. This vaccination strategy uses a booster vaccine from a different platform than the one used to complete the primary vaccination series.
Several studies have evidenced that heterologous boosts induce similar reactogenicity and more immunogenicity than homologous boosts for all combinations. Therefore, back on October 21, 2021, the United States Food and Drug Administration (FDA) authorized the use of mRNA-1273, Ad26.COV2.S, and BNT162b2 COVID-19 vaccines for use as heterologous boosts. Likewise, a systemic review found heterologous priming with BNT162b2 produced robust immunogenicity and tolerable reactogenicity. Yet, more research is warranted to establish optimal combinations, dosing regimens, and long-term safety profiles of heterologous vaccination strategies. To summarize, the current study confirmed the immunogenicity of the nCOV-S(JET) DNA vaccine and showed its potential to elicit a rapid humoral immune response in NHPs.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.