Since its initial emergence in November 2021, the SARS-CoV-2 Omicron variant and its sublineages have significantly influenced the epidemiological landscape of the coronavirus disease 2019 (COVID-19) pandemic. The first Omicron variant, BA.1, was capable of partially evading previously established SARS-CoV-2 wild-type strain (Wuhan-Hu-1)-based immunity due to significant changes in the spike (S) glycoprotein that resulted in the loss of many neutralizing antibody epitopes.
Studies have previously stated that Omicron BA.1 and BA.2 breakthrough infections of people who had previously received immunization with wild-type strain-based messenger ribonucleic acid (mRNA) vaccines or an inactivated viral vaccine were linked to robust neutralizing activity against Omicron BA.1, BA.2, and previous variants of concern (VOCs).
About the study
In the present study, researchers characterized the effect of Omicron BA.4/BA.5 S glycoprotein exposure on the magnitude and breadth of the neutralizing antibody response upon breakthrough infection in vaccinated individuals and in mice upon booster vaccination.
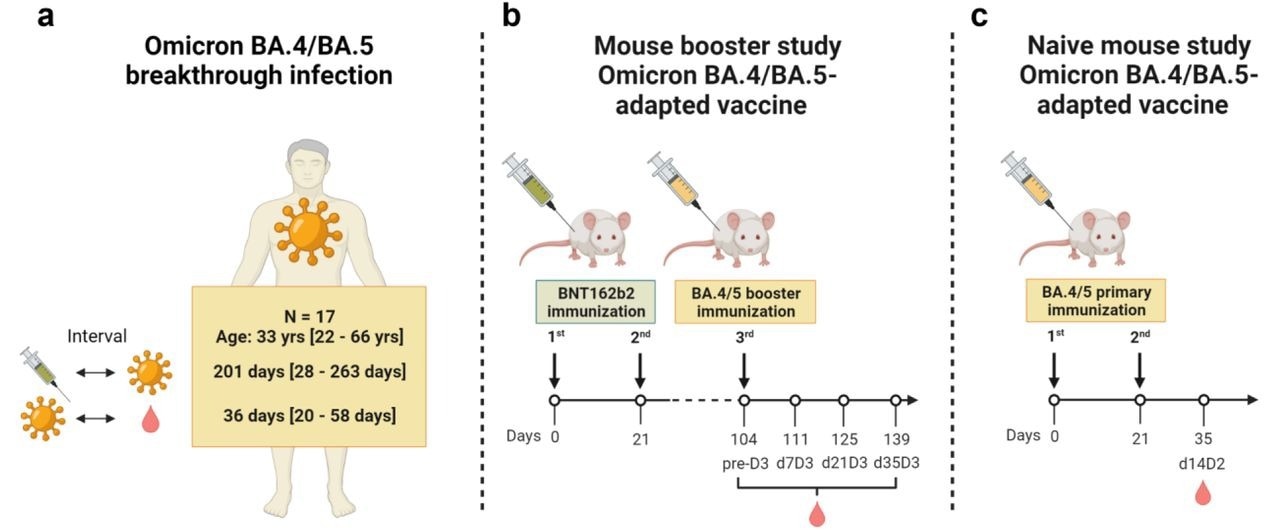 Study design. (a) The effect of Omicron BA.4/BA.5 breakthrough infection on the serum neutralizing activity was evaluated in individuals vaccinated with three doses of mRNA COVID-19 vaccine (BNT162b2/mRNA-1273 homologous or heterologous regimens) who subsequently experienced an infection with Omicron BA.4 or BA.5. The intervals between vaccination, breakthrough infection and sampling are indicated as median/range. (b) Effects of prototypic Omicron BA.4/BA.5-adapted booster vaccines on serum neutralizing activity were investigated in mice vaccinated twice 21-days apart with BNT162b2, followed by a booster dose of BA.4/BA.5-adapted vaccines 3.5 months later. Neutralizing activity was assessed before (pre-D3) and 7, 21, and 35 days after the booster (d7D3, d21D3, d35D3, respectively). (c) Effects of prototypic Omicron BA.4/BA.5-adapted vaccines on serum neutralizing activity were investigated in naïve mice vaccinated twice 21-days apart with BA.4/BA.5-adapted vaccines. Neutralizing activity was assessed 14 days after administration of the second dose (d14D2). Comparably high RNA purity and integrity, and expression of antigens in vitro were confirmed for BNT162b2 and Omicron-adapted vaccines
Study design. (a) The effect of Omicron BA.4/BA.5 breakthrough infection on the serum neutralizing activity was evaluated in individuals vaccinated with three doses of mRNA COVID-19 vaccine (BNT162b2/mRNA-1273 homologous or heterologous regimens) who subsequently experienced an infection with Omicron BA.4 or BA.5. The intervals between vaccination, breakthrough infection and sampling are indicated as median/range. (b) Effects of prototypic Omicron BA.4/BA.5-adapted booster vaccines on serum neutralizing activity were investigated in mice vaccinated twice 21-days apart with BNT162b2, followed by a booster dose of BA.4/BA.5-adapted vaccines 3.5 months later. Neutralizing activity was assessed before (pre-D3) and 7, 21, and 35 days after the booster (d7D3, d21D3, d35D3, respectively). (c) Effects of prototypic Omicron BA.4/BA.5-adapted vaccines on serum neutralizing activity were investigated in naïve mice vaccinated twice 21-days apart with BA.4/BA.5-adapted vaccines. Neutralizing activity was assessed 14 days after administration of the second dose (d14D2). Comparably high RNA purity and integrity, and expression of antigens in vitro were confirmed for BNT162b2 and Omicron-adapted vaccines
The team determined how Omicron BA.4/BA.5 breakthrough infection affected the serum neutralizing activity against SARS-CoV-2 variants of those who had received three doses of the mRNA COVID-19 vaccine. Three cohorts were used as a reference: triple mRNA vaccinated people with an Omicron BA.2 breakthrough infection (mRNA-Vax3 + BA.2) or those having an Omicron BA.1 breakthrough infection (mRNA-Vax3 + BA.1), and triple BNT162b2-vaccinated people who were SARS-CoV-2-naive at the time of sampling. Sera were collected from the biosample collections of BNT162b2 vaccine trials and from a non-interventional investigation examining vaccinated individuals who had had Omicron breakthrough infection.
By measuring 50% pseudovirus neutralization (pVN50) geometric mean titers (GMTs) using pseudoviruses bearing the spike glycoproteins of the SARS-CoV-2 wild-type strain or Omicron BA.1, BA.2, and the BA.2-derived sublineages such as BA.2.12.1 and BA.4/BA.5, serum neutralizing activity was examined. The team also tested SARS-CoV-1 to detect any possible pan-sarbecovirus neutralizing activity. Live SARS-CoV-2 neutralization test (VNT) was employed as an orthogonal test method that examined neutralization during multicycle replication of the genuine virus with immune serum present during the whole test period.
The VOC pVN50 GMTs against the wild-type strain were normalized to enable assessment of neutralization breadth independent of antibody titer magnitude. This allowed the researchers to compare mRNA-Vax3 + BA.4/BA.5 to the reference cohorts having Omicron BA.1 or BA.2 breakthrough infection.
Results
The study results showed that sera wholly neutralized the SARS-CoV-2 wild-type strain and all evaluated Omicron VOCs from the Omicron BA.4/BA.5 breakthrough infection cohort (mRNA-Vax3 + BA.4/BA.5) in the pVNT. The GMTs for the pVN50 against the wild-type strain and the Omicron BA.2 and BA.2.12.1 pseudoviruses were within a 2-fold range. The reduction compared to the wild-type strain was considerable but also lay within a range of two-folds, and neutralization of BA.1 and BA.4/5 was essentially identical to that of BA.2. Additionally, SARS-CoV-1 was markedly lower.
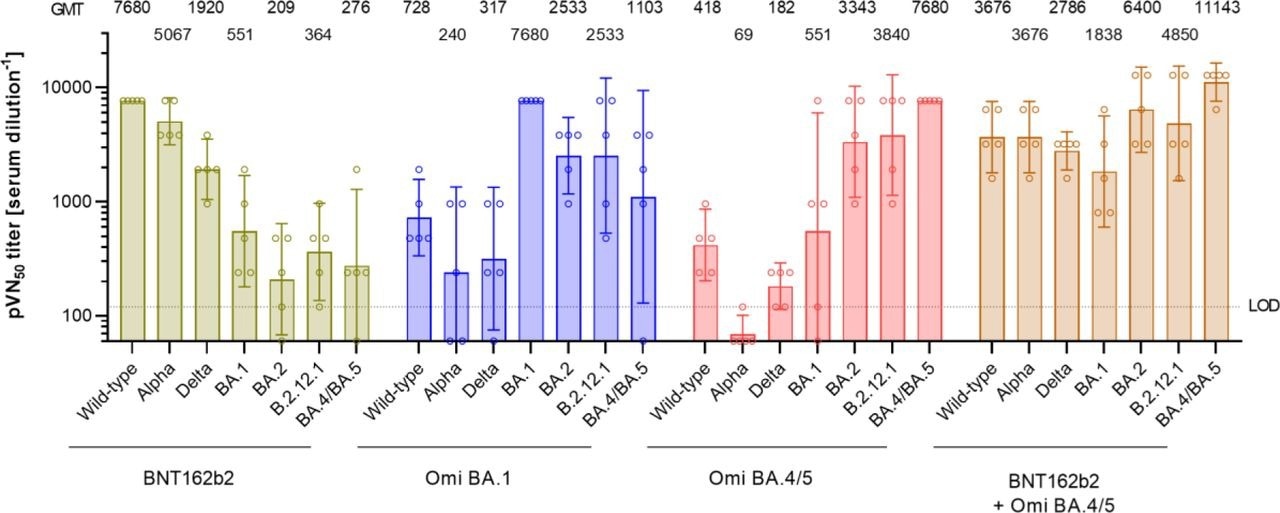
Immunization with an Omicron BA.4/BA.5 S glycoprotein supplemented BNT162b2 mRNA vaccine drives pan-Omicron neutralization in previously unvaccinated mice. Naïve BALB/c mice (n=5) were injected intramuscularly with two doses of either BNT162b2 (1 µg) or the indicated monovalent (1 µg) or bivalent (0.5 µg of each component) Omicron BA.1 or BA.4/5-adapted vaccines, 21 days apart. 50% pseudovirus neutralization (pVN50) geometric mean titers (GMTs) against the indicated SARS-CoV-2 variants of concern (VOCs) in sera collected 14 days after the second vaccination (d14D2). Values above bars represent group GMTs. Error bars represent a 95% confidence interval. Serum was tested in duplicate. For titer values below the limit of detection (LOD), LOD/2 values were plotted.
Although breakthrough infection with Omicron BA.1 was associated with neutralizing the homologous strain, BNT162b2 sera still strongly cross-neutralized Omicron BA.1 and BA.2. With BA.2, neutralization after BA.2 breakthrough infection showed a similar trend without statistical significance. Importantly, when compared to BNT162b23 and mRNA-Vax3 + BA.1, cross-neutralization of BA.4/5 was much stronger in the mRNAVax3 + BA.4/5 cohort. The difference between the mRNA-Vax3 + BA.2 cohort and the cross-neutralization of BA.4/5 was less pronounced.
The mRNA-Vax3 + BA.4/BA.5 cohort maintained cross-neutralization of Omicron BA.1 and BA.2 at considerably larger levels. In conclusion, of all cohorts examined, the BA.4/BA.5 breakthrough infection produced the most effective cross-neutralization across all assessed VOCs.
Omicron BA.4/BA.5 breakthrough sera against BA.2 had VN50 GMT that was comparable to that against the wild-type strain. Significant reductions in BA.4 and BA.1 neutralization occurred. However, they remained within a 2- to 2.5-fold range. The cross-neutralization of Omicron BA.1, BA.2, and BA.4 by mRNA-Vax3 155 + BA.4/BA.5 sera was demonstrated in the GMTs adjusted against the wild-type strain, although cross-neutralization of BA.4 was much less effective in mRNA-Vax3 + BA.1 and mRNA-Vax3 + BA.2 sera. Altogether, the results demonstrated that Omicron BA.4/BA.5 breakthrough infection was linked with broad neutralizing activity against all Omicron VOCs evaluated in both the pVNT and the VNT assay method.
Overall, the study reported that pan-Omicron neutralization, or the robust neutralization of all currently or formerly dominant SARS-CoV-2 Omicron VOCs, is related to neutralization test BA.4/BA.5 breakthrough infection of triple-mRNA vaccinated people.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Exposure to BA.4/BA.5 Spike glycoprotein drives pan-Omicron neutralization in vaccine-experienced humans and mice, Alexander Muik, Bonny Gaby Lui, Maren Bacher, Ann-Kathrin Wallisch, Aras Toker, Carla Iris Cadima Couto, Alptekin Gueler, Veena Mampilli, Geneva J Schmitt, Jonathan Mottl, Thomas Ziegenhals, Stephanie Fesser, Jonas Reinholz, Florian Wernig, Karla-Gerlinde Schraut, Hossam Hefesha, Hui Cai, Qi Yang, Kerstin C Walzer, Jessica Grosser, Stefan Strauss, Andrew Finlayson, Kimberly Krueger, Orkun Ozhelvaci, Katharina Grikscheit, Niko Kohmer, Sandra Ciesek, Kena A Swanson, Annette B Vogel, Oezlem Tuereci, Ugur Sahin, bioRxiv 2022.09.21.508818, DOI: https://doi.org/10.1101/2022.09.21.508818, https://www.biorxiv.org/content/10.1101/2022.09.21.508818v1
- Peer reviewed and published scientific report.
Muik, Alexander, Bonny Gaby Lui, Maren Bacher, Ann-Kathrin Wallisch, Aras Toker, Carla Iris Cadima Couto, Alptekin Güler, et al. 2022. “Exposure to BA.4/5 S Protein Drives Neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in Vaccine-Experienced Humans and Mice.” Science Immunology, November. https://doi.org/10.1126/sciimmunol.ade9888. https://www.science.org/doi/10.1126/sciimmunol.ade9888.