Bats have evolved with unique features such as laryngeal echolocation and flight, with some capable of tolerating viruses such as severe acute respiratory syndrome coronaviruses (SARS-CoVs), Middle East respiratory syndrome CoVs (MERS-CoVs), as well as Marburg and Nipah viruses. Developing robust cell-based bat models could provide a greater understanding of bat viral handling and biology.
Study: Bat pluripotent stem cells reveal unique entanglement between host and viruses. Image Credit: Jezper / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers investigate whether bats could be suitable for viral production.
The Yamanaka reprogramming approach was used based on reprogramming factors such as the sex-determining region Y-box 2 gene, octamer-binding transcription factor 4 (Oct4), cMyc, and Kruppel-like factor 4 (Klf4).
Bat embryonic fibroblast (BEF) cells were isolated from R. ferrumequinum, with the amounts of reprogramming factors altered to activate and block several signaling pathways. In addition, immunostaining and ribonucleic acid (RNA) sequencing (RNA-seq) analyses were also performed.
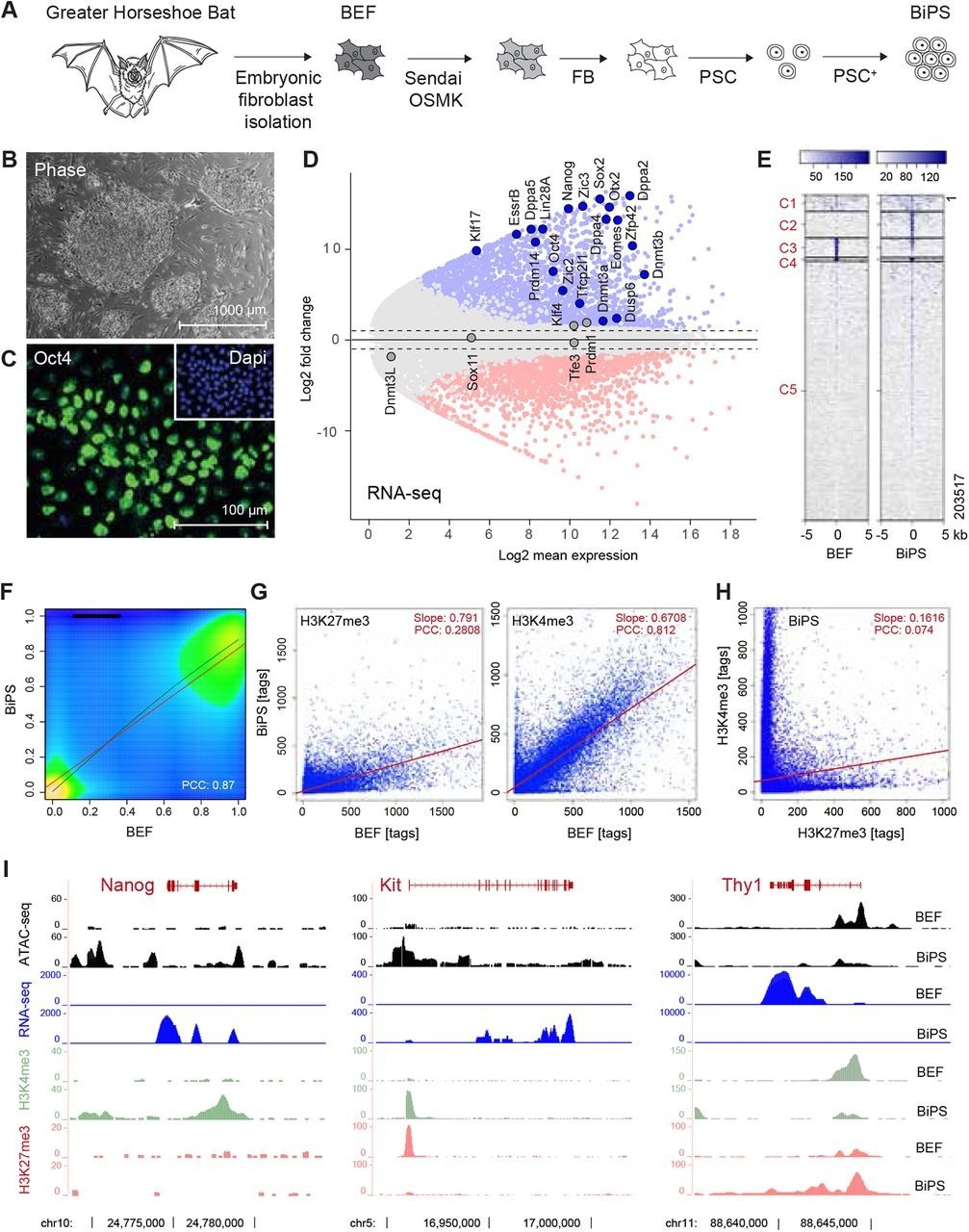
Derivation of pluripotent bat stem cells. (A) Illustration of the bat pluripotent stem cell derivation strategy. BEF, embryonic fibroblasts; OSMK, Oct4, Sox2, cMyc, Klf4; FB, fibroblast medium; PSC, pluripotent stem cell medium; PSC+, PSC with additives. (B) Morphology of established BiPS cell colonies grown on mouse embryonic fibroblasts. (C) Immunofluorescent detection of Oct4 in BiPS cells. (D) MA plot of RNA-seq data illustrating the transcriptional differences between bat embryonic fibroblast (BEF) and pluripotent stem cells (BiPS). Selected genes with known functions in the establishment or maintenance of pluripotency are highlighted. (E) Kmean cluster analysis of ATAC-seq signals obtained from BEF or BiPS cells. C, cluster. (F), Density plot of RRBS results obtained from BEF and BiPS cells. PCC, Pearson correlation coefficient. (G) Scatter plots of histone 3 methylation status at K4 (activating chromatin modification) or K27 (repressing chromatin modification) after ChIP-seq from BEF or BiPS cells as indicated. (H)Scatter plot of H3K4me3 and H3K27me3 in BiPS cells illustrating the occurrence of bivalent chromatin sites in BiPS cells. (I) RNA-seq, ATAC-seq and H3K4me3 or H3K27me3 ChIP-seq signals of selected genes with known roles in reprogramming that are activated (Nanog, Kit) or repressed (Thy1) in BiPS when compared to BEF cells.
The impact of the modified reprogramming method on bat epigenetic molecules and chromatin was assessed using the assay for transposase-accessible chromatin with sequencing (ATAC-seq). Deoxyribonucleic acid (DNA) methylome mapping analysis and chromatin immunoprecipitation and sequencing (ChIP-seq) analyses were also performed. Protocols were optimized to enable bat SC differentiation into the three germ layers, whereas the embryoid body (EB) differentiation assay was performed to assess for pluripotency.
Bat iPSCs (BiPSCs) were subsequently injected into immunosuppressed mice, and embryo-like structures were created from the BiPS. The study protocol was validated by developing BiPS cells of the evolutionally distant Myotis myotis bats.
Comparative transcriptional gene profiling and principal component analysis (PCA) were performed on the Rhinolophus bat species and phylogenetically different mammalian species of mice, humans, dogs, pigs, and marmoset.
Gene ontology analysis was performed to assess the leading-edge gene enrichment for specific biological pathways. Novel pipelines were developed based on the metagenomic classification of stem cell ribonucleic acid (RNA)-sequenced (RNA-seq) data, de novo putative retroviral contig assembly, and genomic mapping for identifying true retroviral reads. In addition, antigen markers related to RNA viruses were explored.
Study findings
A particular reprogramming factor ratio, as well as added fibroblast growth factor-2 (Fgf-2), stem cell factor (Scf), leukemia inhibitory factor (Lif), and forskolin to the culture medium enabled uninhibited BiPSC growth, with homogeneous and tight bat colonies appearing within 14 to 16 days.
BiPSCs expressed the Oct4 pluripotency factor, with a proliferation rate identical to the human PSC proliferation rate. Most cells contained 56 chromosomes and replicated without exogenous reprogramming factors and morphological alterations.
BiPSCs differentiated into the three germ layers, subsequently forming EBs and organoids. RNA-seq analysis showed induced endogenous expression of canonical pluripotency-related genes like SRY-2, Nanog, and Oct4.
However, the genetic profile did not entirely match one pluripotency state. Instead, naive pluripotent state factors such as Klf4 and 17, estrogen-related receptor beta protein (Essrb), transcription factor E3 (Tfe3), and transcription Factor CP2 Like 1 (Tfcp2l1)] were expressed. Co-expressed Tfcp2l1/zinc finger protein (Zic2) and orthodenticle homeobox 2 (Otx2)/Tfe3 and primed/naïve factors were observed.
Chromatin configurational and CpG 191 methylation alterations were observed across the bat genome. ChiP-seq findings showed overlapping between human and bat bivalency genes, although some genes were species-specific.
BiPSCs were reprogrammed transcriptionally and epigenetically. BIPSCs were positive for Paired box protein (Pax6), 213T, and alpha-fetoprotein (AFP) markers for ectoderm, mesoderm, and endoderm, respectively.
The ERAS gene was downregulated, whereas hyaluronidases and ADP ribosylation factors (ARFs) genes were indistinguishable between the groups. The Rhinolophus blastoids showed embryonic structures attached to a flattened trophoblastic epithelial outgrowth and inner cell mass expansion. Myotis bat findings indicated that the study protocol could be applied across different bat species.
PCA analysis showed a distinct group of bat stem cells.However, only eight leading-edge genes showed significant positive selection in R. ferrumequinum, with most genes belonging to unexpected categories. Moreover, CoV disease was the most significantly enriched category in Kyoto encyclopedia of genes and genomes (KEGG) pathways.
Collagen type III alpha 1 (Col3a1) and mucin 1 (Muc1) genes were detected in BiPSCs, thus indicating bat-specific genetic adaptations. Reprogramming revealed endogenous retrovirus (ERV) sequences.
BiPSCs contained several virus-associated endogenized sequences, with regions homologous to the human herpesvirus 4, human respiratory syncytial virus, and a SARS-CoV-2 isolate. R. ferrumequinum genomic sequences resembled those of human CoV 229E and human CoV OC43.
Several retroviral integration sites that were homologous to viruses such as the Mason-Pfizer monkey virus, Koala virus, and Jaagsiekte sheep retrovirus were identified. The genome was homologous to volepox, variola, squirrel pox, monkeypox, and White spot syndrome viruses.
Conclusions
BiPSC sequences were similar to viral genome sequences. Thus, the transcriptionally permissive pluripotency state of bats could be exploited for discovering novel bat virus sequences involved in bat physiology and their virus hosting abilities.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Dejosez, M., Marin, A., Hughes, G. M., et al. (2022). Bat pluripotent stem cells reveal unique entanglement between host and viruses. bioRxiv. doi:10.1101/2022.09.23.509261. https://www.biorxiv.org/content/10.1101/2022.09.23.509261v1.
- Peer reviewed and published scientific report.
Déjosez, Marion, Arturo Marin, Graham M. Hughes, Ariadna E. Morales, Carlos Godoy-Parejo, Jonathan L. Gray, Yiren Qin, et al. 2023. “Bat Pluripotent Stem Cells Reveal Unusual Entanglement between Host and Viruses.” Cell 186 (5): 957-974.e28. https://doi.org/10.1016/j.cell.2023.01.011. https://linkinghub.elsevier.com/retrieve/pii/S0092867423000417.