
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Reduced vaccine efficacy against Omicron
Several randomized clinical trials and real-world studies have reported the efficacy of the first messenger ribonucleic acid (mRNA) COVID-19 vaccines after they became available in 2020. In fact, these vaccines were associated with 97% efficacy in preventing severe illness and deaths due to COVID-19.
Despite the benefits of these vaccines, many people in resource-poor countries could not be vaccinated in a timely manner, which subsequently led to several COVID-19 waves worldwide. The continuous evolution of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, led to the emergence of various viral variants that exhibited superior transmissibility and immune evasive properties.
Previously infected or vaccinated individuals were largely protected against the severe effects of infection, with many of these variants due to natural or vaccine-induced immunity. However, the emergence of the highly immune evasive SARS-CoV-2 Omicron variant reduced the effectiveness of these immune responses following infection with this variant.
To limit the spread and severe effects of infection with this variant, booster vaccine doses were recommended; however, they failed to provide the same degree of protection as the original vaccines against pre-Omicron variants. To overcome the limitations of booster vaccines, bivalent COVID-19 mRNA vaccines comprised antigens of the original SARS-CoV-2 strain, as well as of the Omicron BA.4/BA.5 subvariants, were developed and eventually approved by the United States Food and Drug Administration (FDA).
About the study
The current study involved the recruitment of Cleveland Clinic Health System (CCHS) employees on September 12, 2022, when the bivalent vaccine first became available. Information was collected on the gender, age, job location, and job categorization of all participants.
Any previous diagnosis of COVID-19 before the beginning of the study was reported, along with the pandemic phase when the infection occurred. The study's outcome was time to COVID-19, followed until December 12, 2022.
Study findings
Out of the 51,011 employees included in the study, 20,689 had a history of COVID-19, 12,029 of whom were infected with the Omicron variant. Moreover, 44,592 employees received a single vaccine dose, 42,064 received two doses, 27,254 received three, and 3,858 received four or more doses.
A total of 10.804 employees received the bivalent vaccine, 9,595 of whom received the Pfizer vaccine, and 1,178 received the Moderna vaccine. A total of 2,452 employees tested positive for COVID-19 during the study period.
The risk of COVID-19 was lowest for those infected when the Omicron BA.4/BA.5 variant was the dominant circulating strain. However, the risk of being infected with SARS-CoV-2 was higher for those who received more vaccines.
The bivalent vaccine provided some protection against COVID-19, with an efficacy of about 30%. The protective effect of either bivalent vaccine was 21% for previously infected and vaccinated individuals.
Moreover, people with prior SARS-CoV-2 exposure in the last six to nine months were twice as likely to be re-infected. In contrast, those with previous exposure in the past nine to 12 months were at a 3.5-fold greater risk of contracting COVID-19 than those infected within the last 90 days.
The current study indicates that the bivalent COVID-19 vaccine can provide moderate protection against SARS-CoV-2 infection. However, further research is needed to understand the impact of multiple COVID-19 vaccines on the future risk of infection.
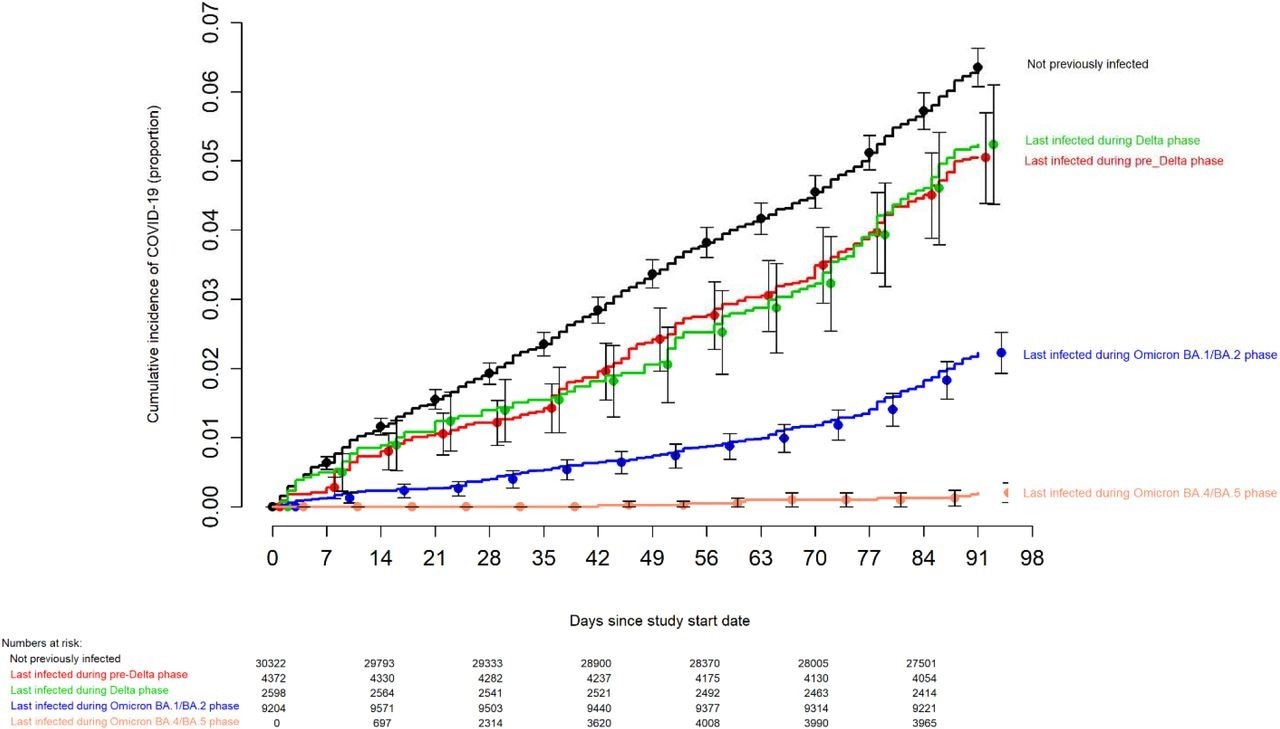 Simon-Makuch plot compares the cumulative incidence of COVID-19 for subjects stratified by the pandemic phase during which the subject's last prior COVID-19 episode occurred. Day zero was September 12 2022, the day the bivalent vaccine began to be offered to employees. Point estimates and 95% confidence intervals are jittered along the x-axis to improve visibility.
Simon-Makuch plot compares the cumulative incidence of COVID-19 for subjects stratified by the pandemic phase during which the subject's last prior COVID-19 episode occurred. Day zero was September 12 2022, the day the bivalent vaccine began to be offered to employees. Point estimates and 95% confidence intervals are jittered along the x-axis to improve visibility.
Limitations
The study has certain limitations. For example, individuals with previously unrecognized infections might have been misclassified, underestimating the bivalent vaccine's protective benefits. Additionally, those receiving the bivalent vaccine were more likely to get tested on symptom appearance, which leads to a disproportionate detection of infections.
Those who received the bivalent vaccine were also more likely to have lower risk-taking behavior concerning COVID-19. Furthermore, the availability of home testing kits might have reduced the detection of infections.
The current study was also unable to differentiate between symptomatic and asymptomatic infections, nor could the researchers determine the impact of the bivalent vaccine on the severity of infection. Notably, no children or immunocompromised individuals were included in the current study, which limits the generalizability of these findings.
Finally, the effect of the vaccine on strains whose antigens are not present within the vaccine is unknown.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Shrestha, N. K., Burke, P. C., Nowacki, A. S., et al. (2022). Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine. medRxiv. doi:10.1101/2022.12.17.22283625. https://www.medrxiv.org/content/10.1101/2022.12.17.22283625v1.
- Peer reviewed and published scientific report.
Shrestha, Nabin K, Patrick C Burke, Amy S Nowacki, James F Simon, Amanda Hagen, and Steven M Gordon. 2023. “Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine,” Open Forum Infectious Diseases April. https://doi.org/10.1093/ofid/ofad209. https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofad209/7131292.