
 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
What is long COVID?
Some people experience ongoing morbidity after recovering from acute coronavirus disease 2019 (COVID-19), a condition that is otherwise known as long COVID. Many researchers have hypothesized that immune dysregulation is responsible for developing long COVID.
Acute SARS-CoV-2 infection has been associated with the production of autoantibodies, particularly among those with severe disease. Several studies have investigated the various immunologic and virologic factors that may contribute to long COVID; however, studies exploring the role of autoantibodies in long COVID remain limited.
About the study
In the present study, researchers determine whether a consistent autoreactivity pattern could be identified in individuals with a history of COVID-19, including many who have been diagnosed with long COVID.
A total of 186 subjects with a confirmed SARS-CoV-2 infection were included in the study. Plasma samples were collected between 60 and 240 days after symptom onset/positive test. In addition, samples from 57 healthy subjects collected before the COVID-19 pandemic served as controls.
Phage immunoprecipitation and sequencing (PhIP-Seq), a proteome-wide approach using T7-phage display assay, immunoprecipitation, and sequencing, was performed on the serum samples.
Participants were also questioned about their quality of life and physical and mental health symptoms. Finally, the team defined six long COVID symptom phenotypes, including cardiopulmonary, neurologic, gastrointestinal, musculoskeletal, upper respiratory, and central nervous system (CNS)-specific symptoms.
Study findings
A distinct autoreactivity pattern was found to explicitly segregate individuals with a history of COVID-19 from SARS-CoV-2-naïve individuals. A single peptide derived from Rho GTPase Activating Protein 31 (ARHGAP31) showed the highest enrichment. There were no differences in enrichment between long COVID subjects and those who had recovered from COVID-19.
Multiple sequence alignment of ARHGAP31 was performed against the whole SARS-CoV-2 proteome, which revealed a region within the viral open-reading frame (Orf1a) polyprotein with high physicochemical similarity to a segment of the ARHGAP31 peptide. This observation corroborates previous reports that anti-SARS-CoV-2 antibodies drive enrichments in the human peptidome.
Further, the researchers compared the distribution of the 20 most enriched peptides between 121 long COVID subjects and 64 recovered subjects without long COVID to examine whether autoantibodies were associated with long COVID. Of these, 17 were present in recovered and long COVID individuals but not in pre-COVID-19 controls.
Peptide enrichments were not significantly different between long COVID and recovered subjects. Notably, peptides from three proteins were only enriched in long COVID individuals.
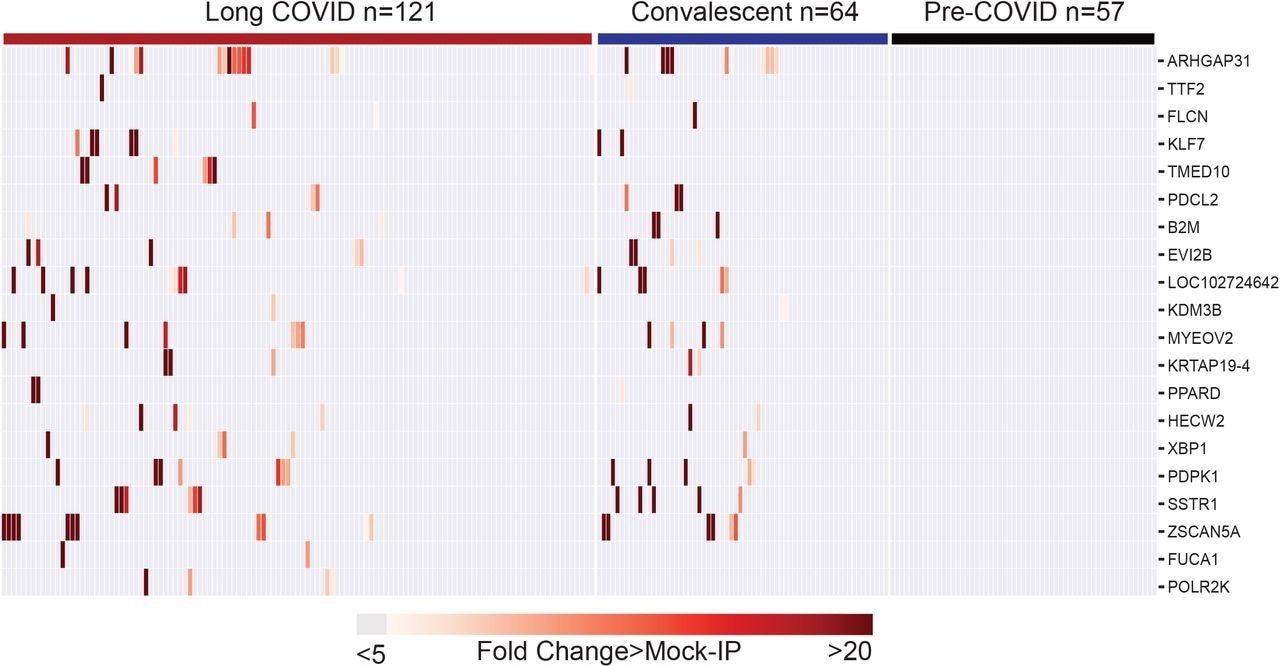
Post-COVID autoreactivities are similarly distributed among Long COVID and controls. Hierarchically clustered (Pearson) heatmaps showing the PhIP-Seq enrichment for the top 20 autoreactivities ranked by logistic regression coefficient in each Long COVID patient, each COVID convalescent patient, and each pre-COVID control.
Sub-group analyses were conducted to ascertain whether autoreactivity was enriched in any of the six symptom phenotypes. The Kolmogorov-Smirnov (KS) test revealed that none of the 20 top peptides were enriched in long COVID.
Notably, the levels of three peptides were significantly elevated in those with upper respiratory or cardiopulmonary symptoms. More specifically, TTF2 and KDM3B levels were more significant in those experiencing cardiopulmonary symptoms, whereas FUCA1 levels were higher in those with upper respiratory symptoms.
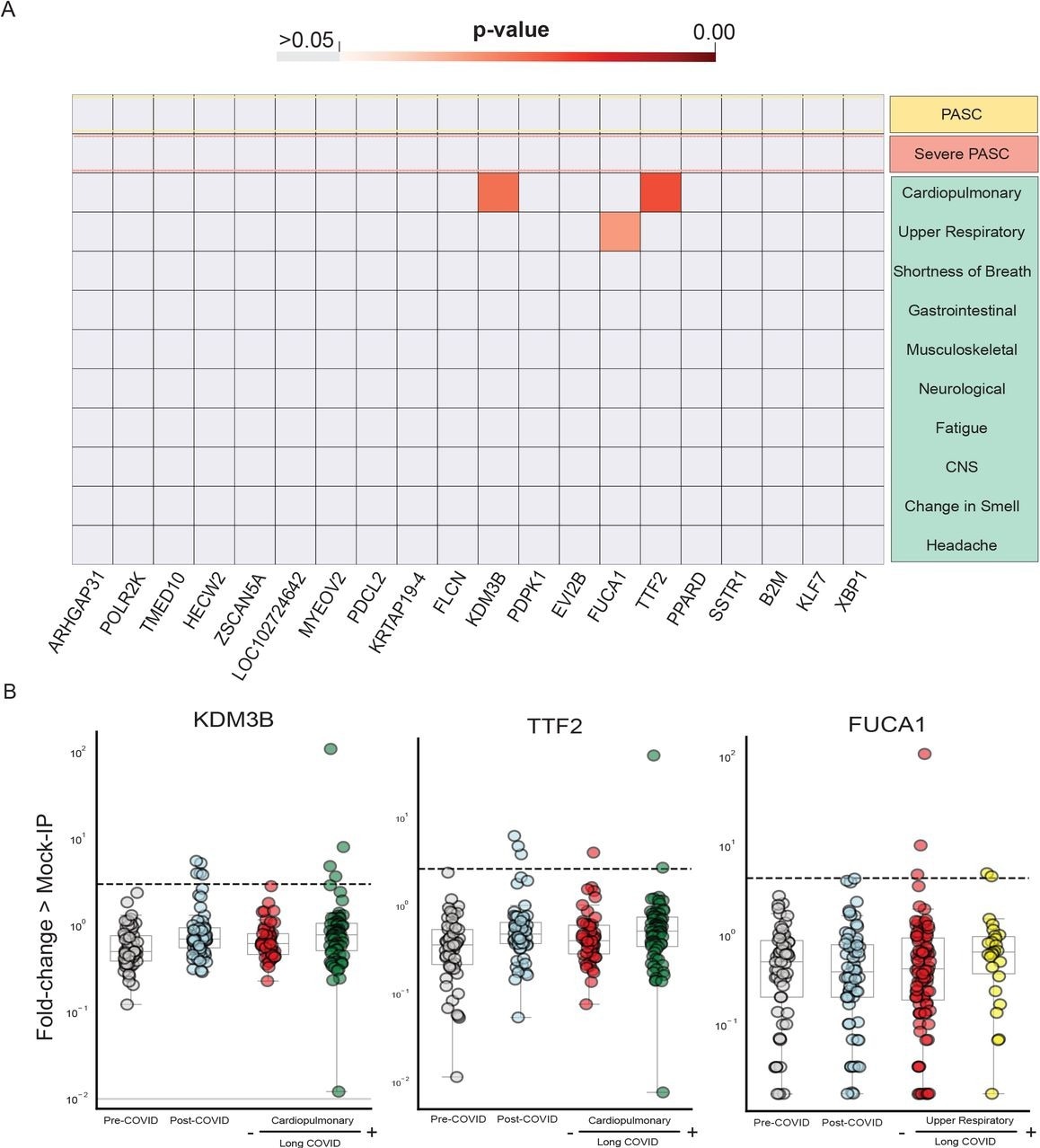
Few significantly increased autoreactivities in Long COVID symptom phenotypes. Heatmap with p-values (Kolmogorov-Smirnov testing) of differences in autoantigen enrichment for all individuals with prior COVID infection with and without additional clinical factors. Top-row compares those with and without Long COVID. Lower rows show subcategories of Long COVID. (B) Stripplots showing the three autoantibodies with statistically significant enrichment in a post-COVID clinical phenotype. Dotted lines show 6 standard-deviations above the mean of pre-COVID signal.
Using logistic regression, the researchers also determined whether peptide enrichments were present exclusively in long COVID. This analysis included six symptom phenotypes, including individuals with generalized anxiety disorder, new depression, poor quality of life, or difficulty concentrating. This study did not yield a set of enriched peptides specific to these groups.
Conclusions
In summary, the researchers applied a proteome-wide approach to evaluate the relationships between clinical phenotypes and autoreactivity.
To this end, a difference in autoreactivity was observed between individuals with COVID-19 and pre-COVID-19 controls. This was due to peptide enrichments from diverse proteins, thus suggesting that the differential enrichment was the result of cross-reactivity with anti-SARS-CoV-2 antibodies.
Taken together, there was no evidence to suggest that autoreactivity contributes to long COVID.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.