The COVID-19 pandemic brought about by the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) led to the disruption of the global economy and the loss of human lives. Studies are still undergoing to understand the molecular mechanisms of SARS-CoV-2 pathogenesis to identify targets for therapeutic interventions. Previous research has highlighted extensive protein misfolding and aggregation associated with COVID-19. There could be three different aspects for the interplay between therapeutic interventions concerning protein aggregation, aggregation of viral proteins might help the virus to hijack the replication machinery of a host cell, viral particles can result in aggregation and misfolding of host proteins which can damage the host organism, and aggregation of viral proteins might be an additional way by which viruses damage host cells.
Certain viral proteins have been reported to form amyloid aggregates involved in viral pathogenesis. Two common examples include protein PB1 of the influenza A virus and protein M45 of murine cytomegalovirus. Additionally, viral capsid proteins can also undergo aberrant aggregation on dysregulation of the functional self-assembly process.
Studies have reported that in SARS-CoV, which is closely related to SARS-CoV-2, the membrane (M) protein can undergo aggregation. Studies have also reported that the C-terminal end of the envelope (E) protein of SARS-CoV comprises an aggregation-prone motif. The transmembrane domain (TMD) of protein E has also been observed to oligomerize to form nonselective ion channels that can act as a viroporin. Additionally, aggregated forms of ORF8b in SARS-CoV have been reported to induce lysosomal damage, endoplasmic reticulum stress, and activation of autophagy.
A new study in the journal Nature Communications aimed to analyze the aggregation propensity in the proteins of both SARS-CoV-2 and SARS-CoV.
 Study: Amyloidogenic proteins in the SARS-CoV and SARS-CoV-2 proteomes. Image Credit: Design_Cells / Shutterstock
Study: Amyloidogenic proteins in the SARS-CoV and SARS-CoV-2 proteomes. Image Credit: Design_Cells / Shutterstock
About the study
The study involved the prediction of aggregation-prone regions (APRs) through four sequence-based methods, FISH Amyloid, FoldAmyloid, AGGRESCAN, and MetAmyl. Thereafter, peptides were prepared for the aggregation assays. Thioflavin T aggregation assay was carried out to analyze the aggregation process in vitro followed by CD spectroscopy, atomic force microscopy (AFM), Raman spectroscopy, high resolution-transmission electron microscopy (HR-TEM), and X-ray diffraction (XRD). Finally, cell viability assays were carried out to understand the impact of non-structural proteins (NSP)11-CoV-2 fibrils on the viability of SH-SY5Y neuroblastoma cells and HepG2 hepatocellular carcinoma cells.
Study findings
The results indicated that the accessory proteins of SARS-CoV-2 were more amyloidogenic as compared to the accessory proteins of SARS-CoV. Among the structural proteins, the membrane (M) and envelope (E) were observed to be more amyloidogenic in SARS-CoV-2 as compared to nucleocapsid (N) and spike (S) proteins. Moreover, NSP4 and NSP6 were observed to have a highly amyloidogenic nature. Additionally, several cleavage sites were observed in the APRs.
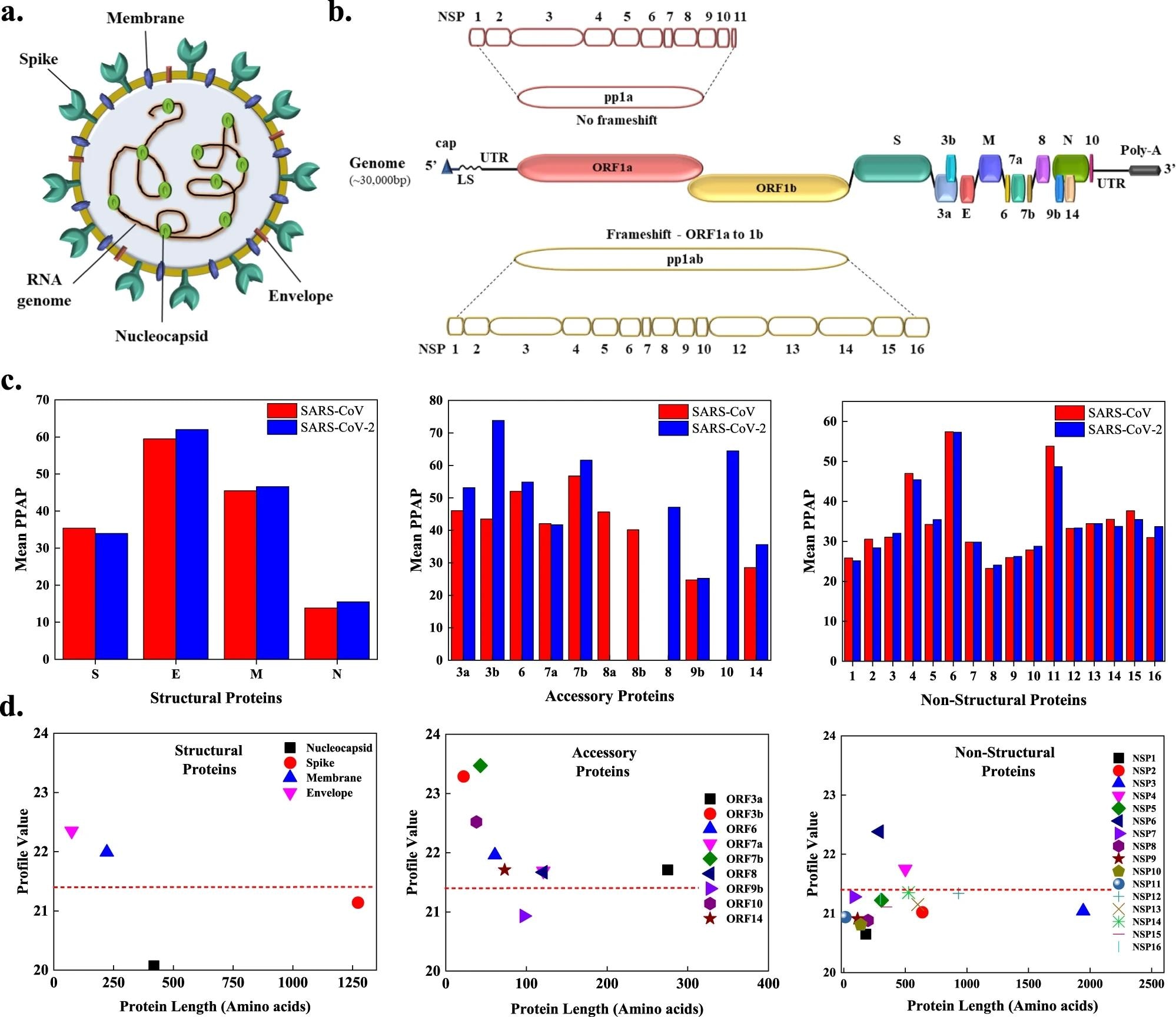 a The SARS-CoV-2 viral particle comprises of positive-sense single-stranded RNA, which is associated with the nucleocapsid protein (N), and three surface proteins, spike (S), membrane (M), and envelope (E), embedded in the lipid bilayer. b The ~30 kbp long genome of SARS-CoV-2 encodes for four structural, nine accessory, and sixteen non-structural proteins (NSPs). c Comparison of mean predicted percentage aggregation propensity (PPAP) calculated using the mean percentage of APRs obtained from four servers (MetAmyl, AGGRESCAN, FoldAmyloid, FISH Amyloid) for SARS-CoV and SARS-CoV-2 structural, accessory, and non-structural proteins. d Average profile value for SARS-CoV-2 proteins obtained from FoldAmyloid analysis of proteins against protein length for structural, accessory, and non-structural proteins. The analysis was done at default settings in the FoldAmyloid server (threshold: 21.4, represented by the red-colored short-dashed line and scale, i.e., the expected number of contacts within 8 Å).
a The SARS-CoV-2 viral particle comprises of positive-sense single-stranded RNA, which is associated with the nucleocapsid protein (N), and three surface proteins, spike (S), membrane (M), and envelope (E), embedded in the lipid bilayer. b The ~30 kbp long genome of SARS-CoV-2 encodes for four structural, nine accessory, and sixteen non-structural proteins (NSPs). c Comparison of mean predicted percentage aggregation propensity (PPAP) calculated using the mean percentage of APRs obtained from four servers (MetAmyl, AGGRESCAN, FoldAmyloid, FISH Amyloid) for SARS-CoV and SARS-CoV-2 structural, accessory, and non-structural proteins. d Average profile value for SARS-CoV-2 proteins obtained from FoldAmyloid analysis of proteins against protein length for structural, accessory, and non-structural proteins. The analysis was done at default settings in the FoldAmyloid server (threshold: 21.4, represented by the red-colored short-dashed line and scale, i.e., the expected number of contacts within 8 Å).
Several APRs were predicted in the S protein as well as the other three structural proteins (M, N, and E) of SARS-CoV-2. 18% amyloidogenic regions were detected in the SARS-CoV-2 N protein, while only 16% were detected in SARSCoV N protein as per AGGRESCAN. Among the accessory proteins, ORF3a, the N-terminal regions of ORF10, ORF8, ORF7a, ORF7b, and ORF6, as well as the C-terminal regions of ORF14, ORF10, ORF9b, ORF8, and ORF7a were reported to have a potential to aggregate.
The results further reported that the 12-residue N-terminal signal sequence (SP-CoV2) that helps the S protein to reach its destination in the viral membrane is an APR and showed aggregation. The two fusion peptides of S protein were also identified as APRs for SARS-CoV and SARS-CoV-2. However, the aggregation kinetics of fusion peptide 2 was slower as compared to fusion peptide 1 of both SARS-CoV and SARS-CoV-2. Moreover, fusion peptides of SARS-CoV-2 were observed to show faster aggregation reactions than the SARS-CoV counterparts.
Furthermore, SARS-CoV-2 ORF10 and NSP6 protein, along with NSP11 of both SARS-CoV and SARS-CoV-2, were reported to show aggregation. Shifts were detected in Raman spectral properties in all the proteins due to structural differences in monomers and aggregates. X-ray diffraction pattern (XRD) results also confirmed amyloid formation by the mentioned peptides. Finally, NSP11-CoV2 aggregates were observed to be toxic for mammalian cells at high concentrations.
Therefore, the current study demonstrated aggregation of a few SARS-CoV and SARS-CoV-2 proteins, which might play an important role in viral pathogenesis. However, further studies are required to understand the role of aggregation of SARS proteins in different pathologies induced by the virus.