Studies have reported an increase in heart rate as a result of anxiety. However, whether tachycardia can result in anxiety is not clear. The team previously reported that ChRmine facilitated the non-invasive neurological modulation of deep cranial circuits, raising possibilities of optogenetic control of cardiac tissues in humans.
 Study: Cardiogenic control of affective behavioural state. Image Credit: Evgeniy Kalinovskiy / Shutterstock
Study: Cardiogenic control of affective behavioural state. Image Credit: Evgeniy Kalinovskiy / Shutterstock
About the study
In the present study, researchers devised a pacemaker for non-invasive optogenetic control of particular heart rhythms during active-type behavior.
Brain-wide activities were screened, and electrophysiological analyses were performed to identify regions of the brain activated by the imposed optogenetic heart rhythms. Cardiomyocyte-limited expression was attained by placing ChRmine under the murine cardiovascular troponin T promoter (mTNT) control, utilizing the AAV9 cardio-trophic serological type.
Wild-type murine animals were utilized to investigate whether systemic ChRmine delivery would permit non-invasive optogenetic controlling of heart rhythms. A 591.0 nm micro-LED was mounted onto wearable fabric vests to deliver light to the skin of the chest wall. To investigate whether cardiac rhythms imposed by the cardiac pacemaker could affect behavior, intermittent ventricular tachycardia of 900 beats per minute for 500 ms at every 1,500 ms was induced optogenetically to simulate non-sustained stress-induced arrhythmias.
Real-time place-preference (RTPP) assays were performed to assess the aversive or appetitive influence of optogenetic cardiac pacing, and elevated plus maze (EPM) assays were performed to assess anxiety-associated behavior. Further, the team investigated if the context-based increase in anxiety-associated behavior would be observed during classical operant tasks using modified Vogel conflict tasks. The willingness of water-limited mice to seek water rewards, although the rewards were associated with shock risks.
Transgenic TRAP2 murine animals, comprising neurons with elevated Fos expression, that could be labeled with the tdTomato neural activation marker, were utilized for whole-brain screening. In vivo electrophysiological experiments were performed using awake murine animals to assess optogenetic cardiac control-imposed neural dynamics at the single-neuron level. Furthermore, optogenetic cardiac inhibition was performed, using blue light-stimulated inhibitory-type channelrhodopsin iC++, to investigate whether the anxiety-generating mechanisms could be modulated for affecting behavior.
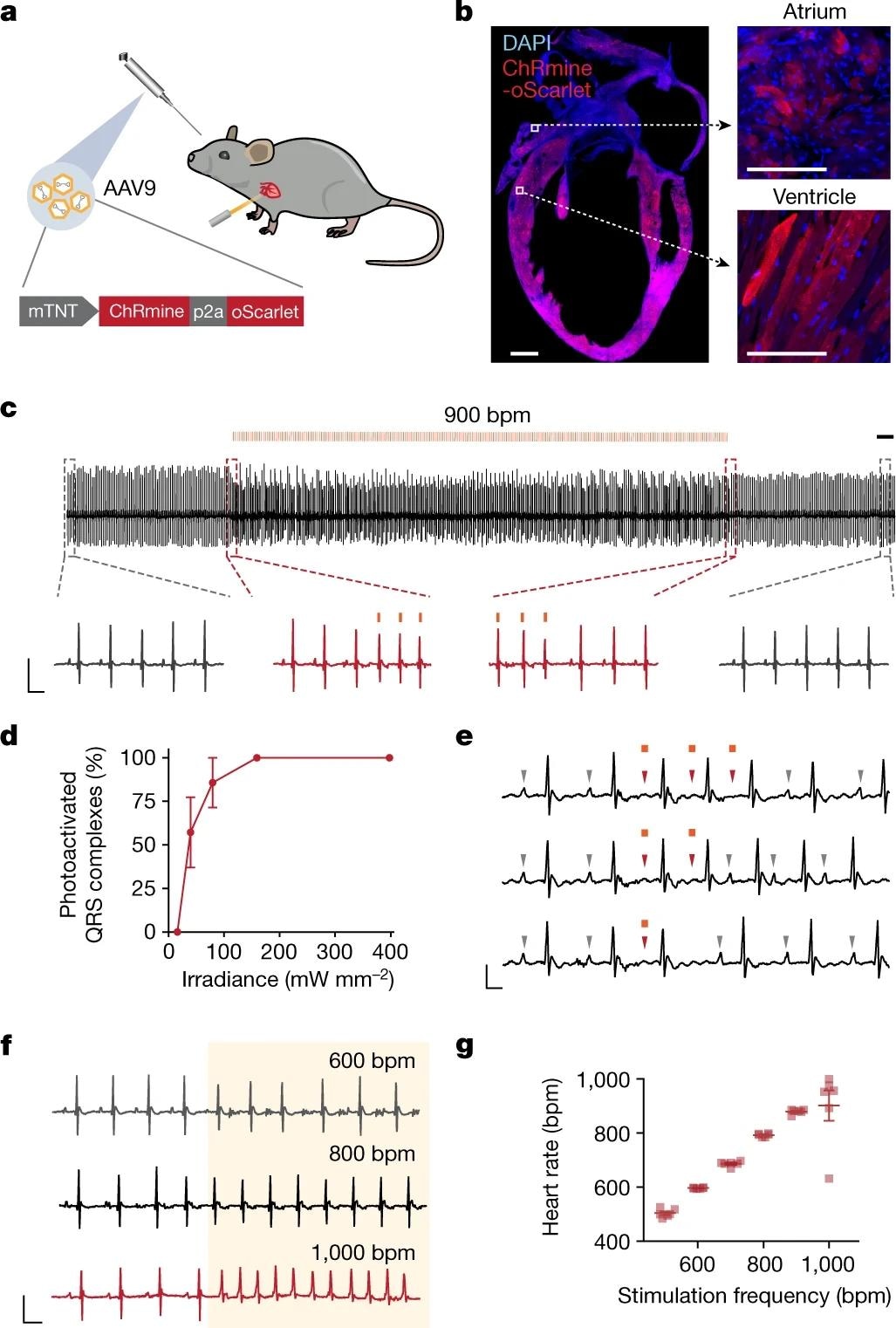
a, Schematic showing the optical control of cardiac rhythm with an external light source enabled by retro-orbital injection of AAV9-mTNT::ChRmine-p2A-oScarlet. b, Confocal cross-section images indicating homogeneous transgene expression of ChRmine-p2A-oScarlet (red) with DAPI staining (blue) in atria and ventricles. Scale bars, 1 mm (main); 100 µm (inset). c, Example electrocardiogram (ECG) trace with optical pacing using 589 nm light delivered at 15 Hz (at 900 bpm) with a pulse width of 10 ms and irradiance of 160 mW mm−2. Scale bar, 500 ms. Inset traces: ECG signal before and after light delivery (grey) and at light onset and cessation (red). Scale bar, 50 ms, 0.5 mV. d, Reliability of photoactivated QRS complexes at 900 bpm as a function of cutaneous optical irradiance (n = 6 mice). e, Example ECG traces of individual 10-ms optical pulses. Grey arrowheads indicate P waves associated with sinus rhythm, which are overridden (red arrowheads) during optical pacing. Scale bar, 25 ms, 0.25 mV. f, Example ECG traces of pacing at 600, 800 and 1,000 bpm. Scale bar, 50 ms, 0.5 mV. The shaded area indicates the period of illumination at the specified frequency at 100% duty cycle. g, Characterization of optical pacing fidelity, showing stimulation frequency versus ECG-measured heart rate (n = 6 mice). All ECG measurements were performed in anesthetized mice. Data are mean ± s.e.m.
Results
Optically induced intermittent ventricular tachycardia potently increased anxiety-associated behavior, crucially in risky-type contexts only, indicative of brain (central) and body (peripheral) involvement in emotional development. The posteriorly located insular cortical region (pIC) was identified as a probable regulator of bottoms-up heart interoceptive-type processing, the optogenetic blockade of which suppressed anxiety-associated behavior initially induced by optogenetic cardiovascular pacing.
Infecting cardiomyocytes with AAV9-mTNT::ChRmine-2A-oScarlet resulted in light-activated contractions with an irradiance of 0.10 mW per mm2. Retro-orbital AAV9 injections led to a limited but homogeneous expression of ChRmine in cardiomyocytes, with no off-target expression in other cardiac cells such as fibroblasts, neuronal ganglia, and other organs.
Combining a molecular approach with accessible electronic devices facilitated sustained and non-invasive cardiac pacing at pre-determined rhythms appropriate for behavior assessment using freely mobile mice. In the EPM assay, mice exhibited limited exploration of the open (exposed) arms of the apparatus after optical pacing. After introducing shocks, optically paced mice suppressed or abolished water-seeking, with an increase in apprehension.
Optical pacing increased Fos messenger ribonucleic acid (mRNA) levels in the pIC and the brainstem, especially in the nucleus tractus solitarius sensory neurons and noradrenergic neuronal cells of the locus coeruleus, associated with stress and arousal. Optogenetic pacing activated the pIC, the blockade of which, alone, was inadequate in inducing anxiolysis. Intermittent cardiac stimulation at 660.0 bpm did not lead to anxiety-like behavior, indicating that the changed rate, instead of the external nature of the timing of heart contractions, was essential.
The attenuation of anxiogenic behavior during optogenetic pIC inhibition indicated casual involvement of the insular region in assimilating sensory cardiac data with the contextual evaluation of environmental risks involved in generating patterns of adaptive behavior. The findings indicated the involvement of the insula in assessing consummatory and interoceptive internal states for instructing relevant behavior-associated responses.
The findings indicated that cell-specific, temporally accurate, and non-invasive organ physiology perturbations are possible among mammals. The devised method, requiring no special surgery or optoelectronics, could be applied to various physiological organ systems in the human body, unraveling novel opportunities for exploring the complicated mechanisms of health and disease.
Overall, the study findings showed that peripheral and central pathways must be elucidated to improve understanding of emotion and behavior’s origin. In addition, the study findings showed a generalizable method for temporally accurate and non-invasive functional assessments of joint organ-wide physiological interactions in the cellular targets during anxiety-like behavior.