Small amounts of nanometer-thin metal-organic layers efficiently protect red blood cells during freezing and thawing, as a team of researchers writing in the journal Angewandte Chemie has discovered. The nanolayers, made from metal-organic frameworks based on the metal hafnium, prevent ice crystal formation at very low concentrations. This effective novel cryoprotection mode could be used to develop new and more efficient cryoprotectants for the biosciences.
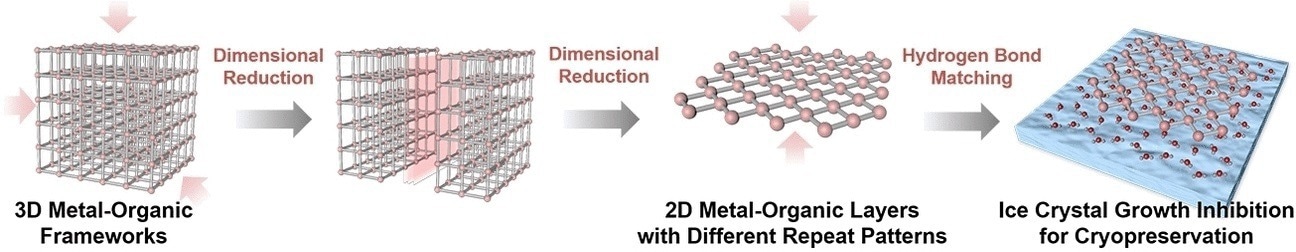
Image Credit: Angewandte Chemie
Cryoprotectants prevent ice crystals from forming when samples are frozen. Growing crystals can damage delicate cell membranes and cell components and disrupt cell integrity. Some solvents or polymers make good cryoprotectants; they keep ice crystal formation in check by binding water molecules and disrupting their ordered assembly during ice formation.
Synthetic chemistry has yet more tricks up its sleeve for targeting and influencing ice formation in a more effective way. Metal-organic frameworks (MOFs) are three-dimensional crystalline networks of metal ions linked by organic ligands. These ligands can be tailored to bind small molecules such as water, allowing the assembly of the water molecules into ice crystals to be very precisely tuned.
Wei Zhu from the South China University of Technology in Guangzhou (China) and colleagues have now discovered that as MOFs based on hafnium and organic ligands become thinner, their ability to bind and influence water molecules increases, mainly because more ligand sites are available. The team therefore developed a method for controlled deconstruction of the three-dimensional metal-organic frameworks until only two-dimensional thin nanolayers remain.
To test the suitability of their hafnium-MOLs (MOL stands for metal-organic layer, to distinguish them from three-dimensional MOFs) as cryoprotectants, the team froze red blood cells, a type of cell that needs to be stored in large numbers for medical purposes but is easily destroyed by ice crystal formation. Compared to the hydroxyethyl starch (HES), which is commonly used as a cryoprotectant, hafnium MOLs showed excellent cryoprotection at a minimal concentration of less than 0.1%, whereas the HES solutions are typically used at concentrations of up to 30%.
Zhu and the team explained that the MOLs are so effective because the irregular two-dimensional structure of the ligands binding the water molecules prevents the formation of regular ice crystal nuclei. The team suggest that the dimensional reduction of MOFs is an interesting new perspective for obtaining highly efficient cryoprotectants.
Source:
Journal reference:
Lei, Q., et al. (2023). Dimensional Reduction of Metal−Organic Frameworks for Enhanced Cryopreservation of Red Blood Cells. Angewandte Chemie. doi.org/10.1002/anie.202217374.