Apart from chronological age, the biological age in adults is associated with health and resilience in the face of disease. Children might also be at risk of health complications if their biological age exceeds their chronological age. A new research paper published in the journal Computational and Systems Biology, Epidemiology and Global Health explores how child development relates to two new candidate markers of biological age.
Geroscience is based on the hypothesis that biological aging causes the breakdown of bodily processes, resulting in multiple disease conditions dismissed as part of old age. Many scientists have tried to identify markers of biological aging to provide a foundation for preventive interventions that could slow this downward trend and preserve health in later life.
Telomere length is a commonly used marker of biological age, directly reflecting one of the fundamental Hallmarks of Aging. It is now being supplemented by high-throughput omics, examining epigenetic, transcriptional, translational, and metabolic pathways that change more or less uniformly with molecular-level aging.
These are unified to derive biological clocks and global assessments of cellular aging. Accelerated aging, which is the difference between predicted and chronological age, is a marker of age-related diseases and mortality risk.
One such type of clock is the Horvath clock and others, all based on DNA methylation. These have been further developed by integrating other clinical biomarkers and mortality data to yield second-generation clocks.
Child development and aging are related in a cause-effect manner, according to the developmental origin of health and disease hypothesis. Healthy development thus builds up a greater stock of physical, cognitive, and mental health, which is reflected in a more extended period of healthy living in later years. This is explained by the ability to sustain one's capabilities at suprathreshold levels for a longer period following the onset of late-adulthood-related decline.
In keeping with this postulate, Horvath's DNA methylation clock shows a standard, unidirectional trend of change in the level of methylation throughout life but faster in childhood. Some researchers consider that accelerated DNA methylation aging may thus be beneficial in building up biological capital for a longer period of good health as life goes on.
This is in contrast with the significance of accelerated DNA methylation age in adults, which could reflect reduced biological capital, being the result of increased efforts to repair the wear and tear on the cells caused by external and intrinsic factors operating during daily life.
Telomere shortening is considered an unfavorable marker, a response to early cell damage that may persist in adulthood. Biological clocks based on omics are new in the field but may reflect actual biological age better because they measure the actual life processes that are expressed as the phenotype, unlike the telomere length and DNA methylation clocks.
The current study included about 1,200 children between the ages of 5 and 12 years. The multinational study provided data from participants in the UK, France, Spain, Norway, Lithuania, and Greece. All were European and part of the Human Early Life Exposome (HELIX) study.
The researchers determined how biological age indicators were related to developmental outcomes in children, such as growth, fat deposition, cognition, behavior, lung function, and the time of puberty.
They used multiple biological clocks, including those based on telomere length, DNA methylation, and gene expression, as well as those derived from the levels of various proteins and metabolites. In particular, they tested the transcriptome- and immunometabolic-based age for consistency with other clocks.
The immunometabolic clock incorporates 135 markers of metabolic and inflammatory function, both being the final result of gene expression. The transcriptome clock was composed of almost 1,500 genes. These clock-derived markers were expressed in terms of the difference between the predicted and chronological age (Δ age).
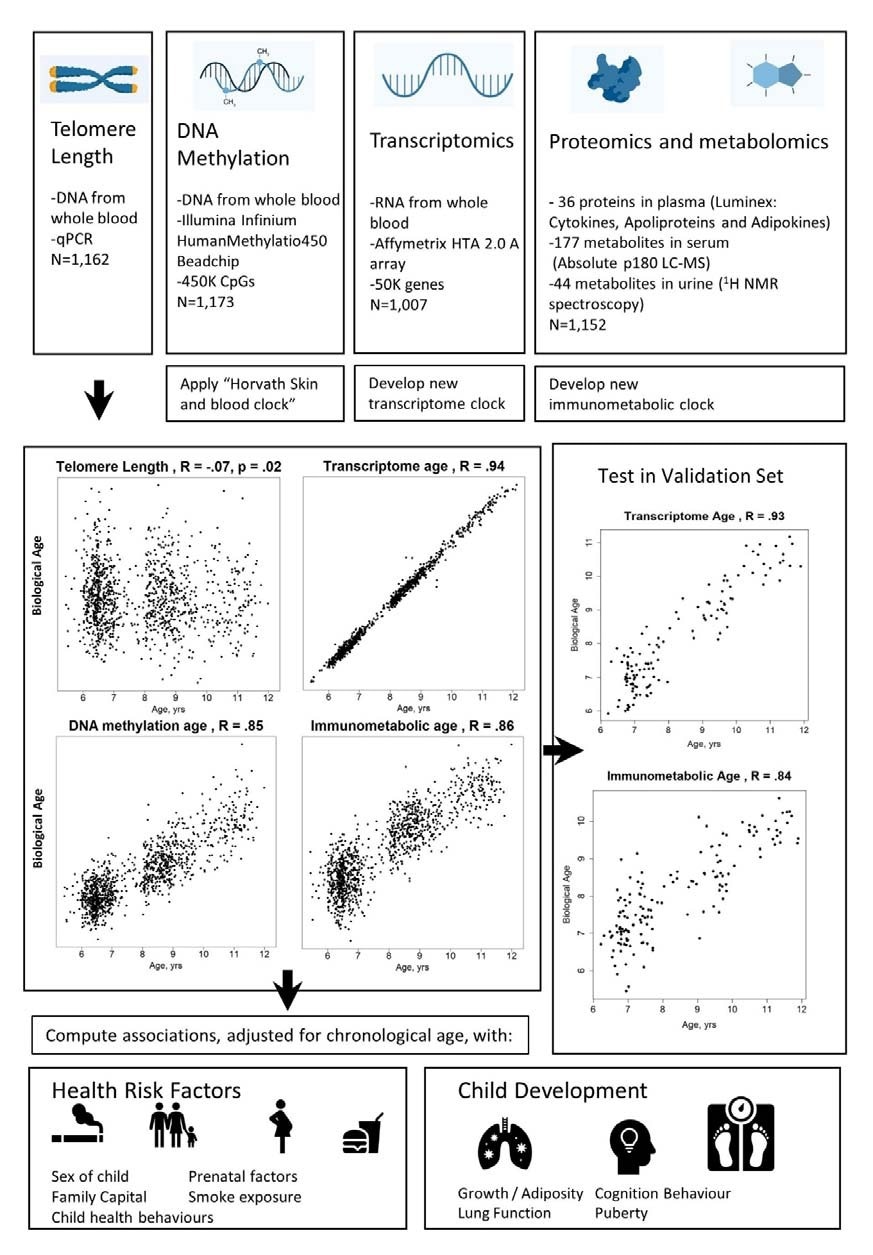
Study design schematic.
What did the study show?
The researchers were able to predict the chronological age correctly using two new biological clocks, the transcriptome, and immunometabolic clocks.
The biological age markers showed only weak associations with each other, confirming that they reflect different aspects of aging.
A higher birth weight was linked to a higher Δ age by immunometabolic markers. Family affluence was linked to longer telomere lengths.
Girls had longer telomeres than boys. This is well-known to be the case in adult life, but its presence in children indicates a biological foundation, which mediates increased risk when males are faced with other health risk factors.
Boys with shorter telomeres and higher transcriptome Δ age were more likely to have higher BMIs, while transcriptome and DNA methylation Δ age in boys were linked to poorer behaviors. Conversely, transcriptome and immunometabolic Δ age in girls was more strongly associated with better attentiveness.
"Given observed sexual dimorphism in both developmental rates and biological age measures, it may be unsurprising that relationship between biological age and development also differs between the sexes."
Meanwhile, the DNA methylation Δ age was increased in association with smoke exposure during gestation by passive or active maternal smoking. It was lower with children from socially secure families, while transcriptome Δ age was higher.
All the markers of biological age are linked to higher body mass index (BMI) and fat mass. Conversely, every marker except for telomere length was linked with increased height.
A higher immunometabolic Δ age indicated higher odds for improved working memory, with less inattentiveness, compared to increased inattention with a higher DNA methylation Δ age. The latter was also linked to greater externalizing behaviors and shorter telomeres.
What are the implications?
The scientists were able to derive two new measures of biological age and test two established markers. Though poorly correlated with each other, all markers were linked to increased weight gain and fat deposition.
The transcriptome clock was found to correlate well with the predicted chronological age, even at the second assessment six months later. Thus, gene expression is closely associated with age. However, this clock correlated poorly with developmental outcomes other than fat deposition and growth.
This indicates the need for clocks that reflect clinical situations rather than merely the chronological age.
BMI is a persistent marker of accelerated biological aging and emphasizes the role of dysfunctional metabolism in age-related physiological decline. Interestingly, it was the single risk factor for poor health to be identified in association with accelerated aging measured by five biological clocks.
"Here we show that the link between BMI and multiple dimensions of accelerated ageing is also apparent in children." All Aging Hallmarks bear a close correlation with energy-nutrition balance, with a high-fat mass being consistently linked to premature death.
This could be because the body prioritizes anti-aging pathways of cell repair when faced with limited nutrient availability, in preference to growth and metabolism pathways. Increased fat deposition is also a source of inflammation and oxidative stress, associated with the shortening of telomeres and increased rates of DNA methylation.
In addition, children with accelerated DNA methylation age and immunometabolic age tended to be taller than others. This corroborates earlier studies.
While this could indicate a better growth environment, more evidence is required to rule out an adverse facet of increased height gain. For instance, rapid gain in height could increase wear and tear on cells, and some studies indicate that with excessive early height, life expectancy drops.
The immunometabolic age was also linked to more mature cognitive skills, such as working memory and attentiveness. This may indicate better immune and metabolic maturity and better overall development.
As with adults, children also experience biological aging across a broad spectrum. Increased fat deposition correlates with faster biological aging. Again, while accelerated DNA methylation age and telomere attrition in children could be potentially detrimental to their future health, advanced immunometabolic age might benefit the child's development in some respects.