In addition to movement disorders or motor symptoms, PD causes dementia and cognitive changes, which worsen the quality of life of affected individuals. Many effective disease-altering therapies for neurodegeneration are current available; however, the treatment of PD-induced neurodegeneration requires detecting changes at the earliest possible stages.
Previously, doctors used magnetic-resonance imaging (MRI) to monitor PD progression; however, this modality is relatively insensitive in detecting the early stages of dementia in PD. To this end, MRI relies on measuring the volume or thickness of cortical regions alone, whereas other measures that are sensitive to changes in brain tissue composition, such as brain tissue iron, are more effective for detecting and tracking neurodegeneration associated with PD.
In PD, iron accumulates in the basal ganglia and substantia nigra (SN). The excessive presence of iron in these brain areas causes an increase in reactive oxygen species (ROS) that interact with pathological proteins, alpha-synuclein, and beta-amyloid.
Using QSM, researchers have previously demonstrated that magnetic susceptibility was related to cognitive and motor severity in PD. To date, QSM has only been evaluated in cross-sectional studies; therefore, it remains unknown whether this approach could monitor PD-related neurodegeneration longitudinally in the whole brain or pre-selected regions of interest (ROIs) over time.
About the study
In the current three-year follow-up longitudinal study, researchers apply an optimized longitudinal QSM approach to detect neurodegeneration changes in brain regions of PD patients without dementia at study initiation.
At the study baseline, 107 patients with early to mid-stage PD between 49 and 80 years of age who were diagnosed within the past 10 years were included. The study enrollment began in October 2017 and ended in December 2018. In addition, 37 age-matched controls were recruited to participate, including unaffected patient spouses.
Clinical assessments of all participants at baseline and follow-up after three years were performed, with a follow-up interval average of about 38.5 months. The neuropsychological evaluation of all participants encompassed five cognitive domains including word recognition and verbal fluency.
Z-scores were used to calculate composite cognitive scores. Additionally, all participants were assessed for motor functions, smell, depression and anxiety, and rapid eye movement (REM) sleep behavioral disorder.
At both time points, MRI measurements comprising susceptibility- and T1-weighted scans were obtained. After QSM reconstruction and spatial standardization of susceptibility maps, absolute QSM data was used for QSM statistical analyses of the whole brain.
Post-hoc ROI analyses were also performed to determine any interactions between motor and cognitive severity and signed susceptibility in PD patients. The ROIs for this analysis included substantia nigra pars compacta (SNpc) and pars reticulata (SNpr), red nucleus, caudate nucleus, putamen, globus pallidus, hippocampus, nucleus basalis of Meynert (NBM), insular cortex, lateral, medial orbitofrontal cortex, and dentate nucleus.
Voxel-based morphometry (VBM) analyses allowed the researchers to compare the QSM performance and conventional atrophy-based measures in predicting clinical severity.
QSM is an effective tool for monitoring PD progression
At baseline, PD patients who exhibited greater cognitive decline after 38 months follow-up had higher magnetic susceptibility values in the right basal forebrain, temporal cortex, and putamen. Similarly, a rise in baseline magnetic susceptibility values in the basal ganglia, SN, insular cortex, red nucleus, and dentate nucleus were related to greater motor severity in PD patients at follow-up.
After longitudinal follow-up, while basal ganglia and SN continued to exhibit increased magnetic susceptibility values in regards to greater motor severity in whole-brain and ROI analyses, the hippocampus and basal forebrain exhibited higher susceptibilities related to poorer cognition.
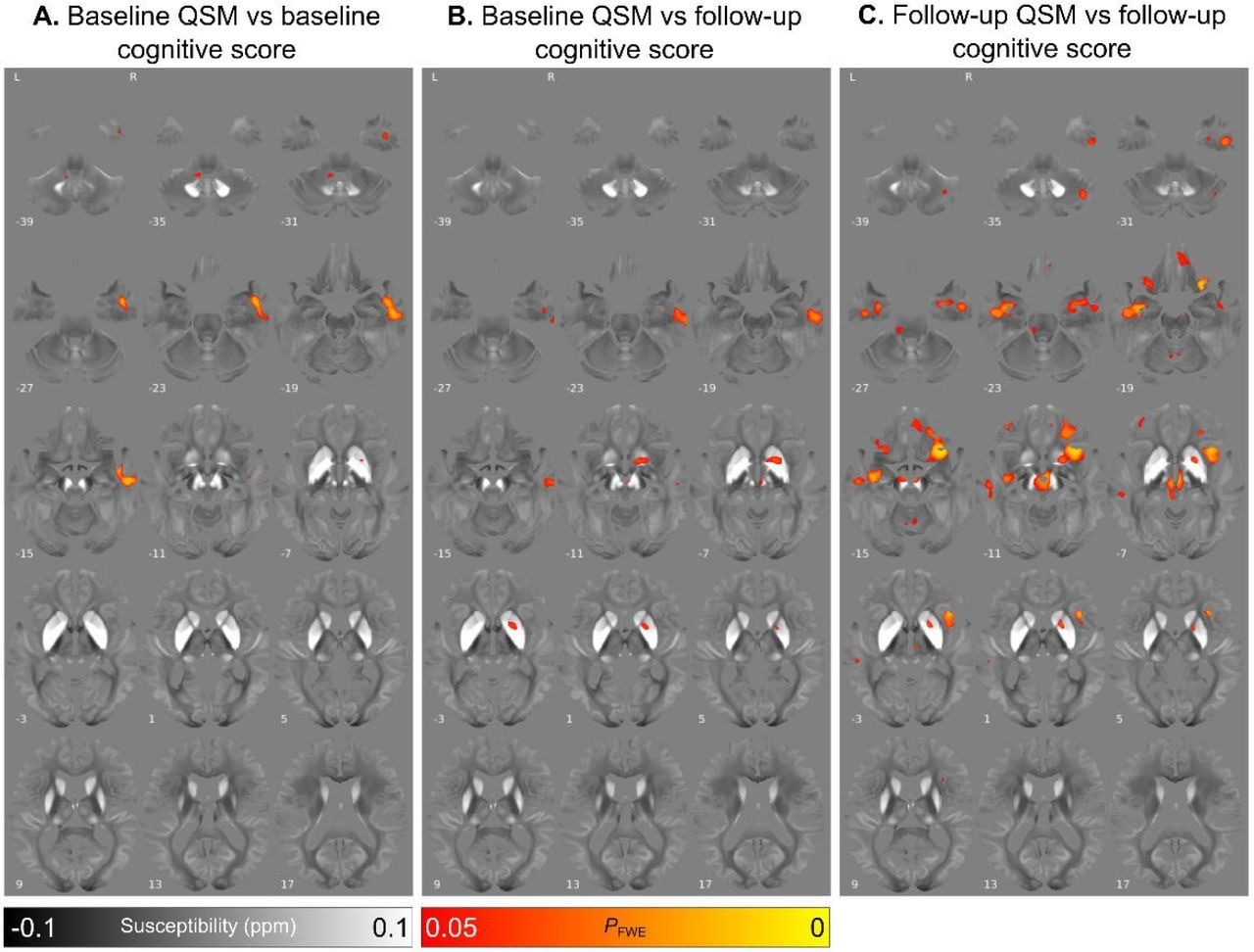 Relationship between increased absolute magnetic susceptibility and declining cognitive ability over the Parkinson’s disease course, in a whole brain analysis. A. Association between baseline susceptibility and composite cognitive score at baseline, adjusted for age at baseline and sex. B. Association between baseline susceptibility and composite cognitive score at 3-year follow-up, adjusted for age at baseline, sex and time between scans. C. Association between susceptibility at 36-month follow-up and composite cognitive score at 3-year follow-up, adjusted for age at follow-up and sex. Results are overlaid on the study-wise QSM template in MNI space, and numbers represent axial slice location in MNI space. Left side is shown on the left. Red/yellow clusters represent voxels where a significant relationship was seen at FWE-corrected P<0.05.
Relationship between increased absolute magnetic susceptibility and declining cognitive ability over the Parkinson’s disease course, in a whole brain analysis. A. Association between baseline susceptibility and composite cognitive score at baseline, adjusted for age at baseline and sex. B. Association between baseline susceptibility and composite cognitive score at 3-year follow-up, adjusted for age at baseline, sex and time between scans. C. Association between susceptibility at 36-month follow-up and composite cognitive score at 3-year follow-up, adjusted for age at follow-up and sex. Results are overlaid on the study-wise QSM template in MNI space, and numbers represent axial slice location in MNI space. Left side is shown on the left. Red/yellow clusters represent voxels where a significant relationship was seen at FWE-corrected P<0.05.
In the longitudinal analyses, all baseline susceptibility changes predictive of cognitive deficits and motor decline in PD patients became robustly associated and more widespread across other relevant brain regions. However, the ROI analysis did not identify marked magnetic susceptibility value increases over time, even in relevant brain regions.
Prior longitudinal studies using QSM to evaluate PD patients failed to show harmonious temporal increases in signed susceptibility values and focused more on motor outcomes, rather than cognitive declines.
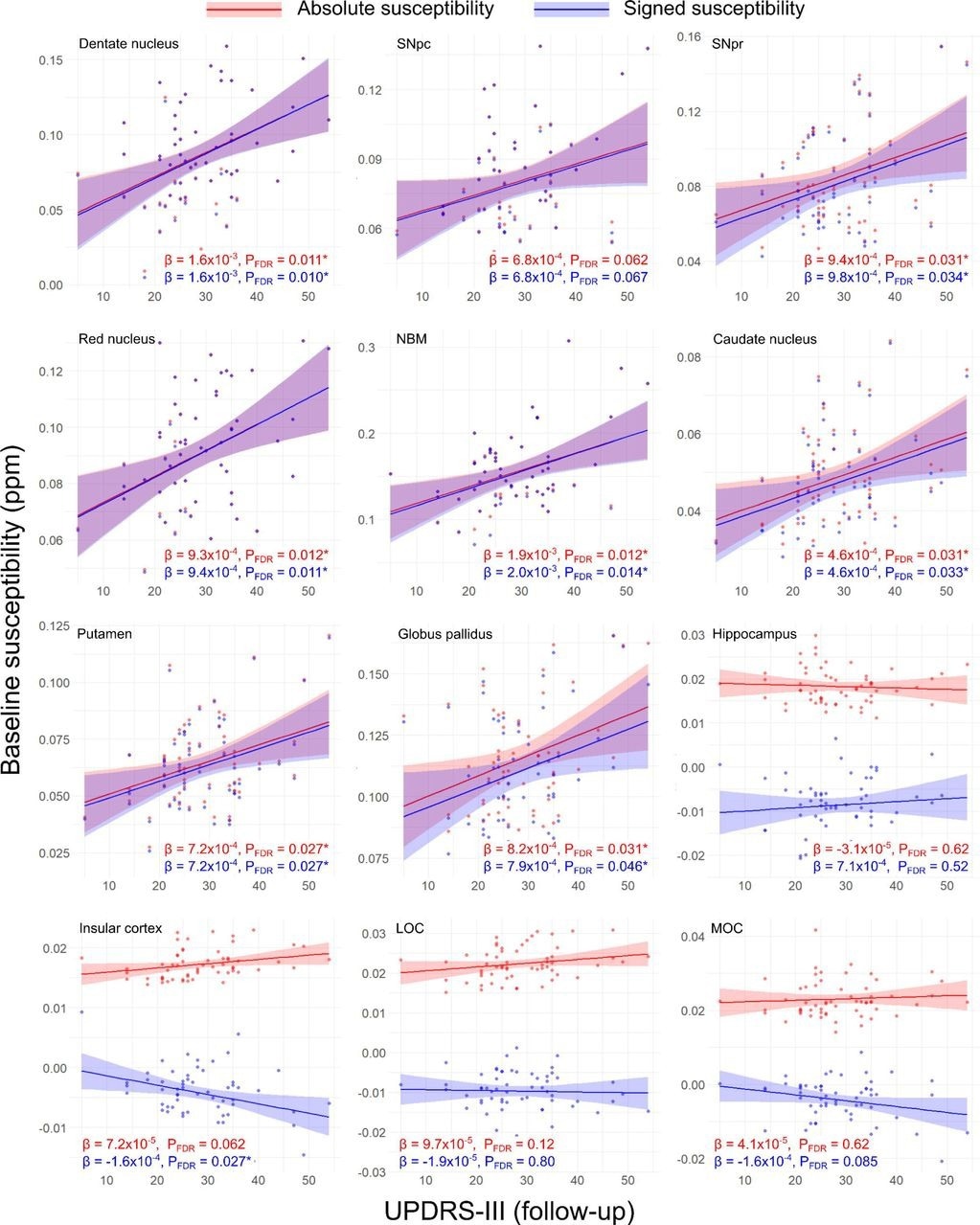 Regional relationships between baseline magnetic susceptibility and follow-up motor severity score in PD. Data and statistics relating to absolute magnetic susceptibility are shown in red, and those relating to signed susceptibility are shown in blue. Results are adjusted for age at baseline, sex and time between scans. FDR-corrected p-values (PFDR) are presented, with asterisks indicating significant interactions at PFDR < 0.05. β is the linear model coefficient associated with MDS-UPDRS-III score. SNpc/pr = substantia nigra pars compacta / pars reticulata; NBM = nucleus basalis of Meynert; MOC = medial orbitofrontal cortex; LOC = lateral orbitofrontal cortex; MDS-UPDRS-III = Movement Disorders Society Unified Parkinson’s Disease Rating Scale part III.
Regional relationships between baseline magnetic susceptibility and follow-up motor severity score in PD. Data and statistics relating to absolute magnetic susceptibility are shown in red, and those relating to signed susceptibility are shown in blue. Results are adjusted for age at baseline, sex and time between scans. FDR-corrected p-values (PFDR) are presented, with asterisks indicating significant interactions at PFDR < 0.05. β is the linear model coefficient associated with MDS-UPDRS-III score. SNpc/pr = substantia nigra pars compacta / pars reticulata; NBM = nucleus basalis of Meynert; MOC = medial orbitofrontal cortex; LOC = lateral orbitofrontal cortex; MDS-UPDRS-III = Movement Disorders Society Unified Parkinson’s Disease Rating Scale part III.
Conclusions
The current longitudinal study used a whole-brain approach and more sensitive measure of cognitive severity to determine that magnetic susceptibility could more effectively predict cognitive and motor severity changes over three years of PD progression. However, robust regional increases in susceptibility were observed, thus suggesting that this approach might be more limited in predicting disease changes over time.
The whole-brain analysis results indicated that increased baseline susceptibility in the right NBM was predictive of poorer cognitive performance at the three-year follow-up that involved bilateral hippocampi, temporal cortex, red nucleus, and orbitofrontal cortices.
Future studies are needed to combine imaging modalities sensitive to myelin and the accumulation of pathological proteins, such as tau, to distinguish between the specific tissue changes observed in PD-related dementia and how these relate to measures of clinical severity.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Thomas, G. E. C., Hannaway, N., Zarkali, A., et al. (2023). Magnetic susceptibility is predictive of future clinical severity in Parkinson's disease. medRxiv. doi: 10.1101/2023.07.12.23292555.