RBCs primarily transport oxygen and possess immune functions; while immature RBCs in newborns suppress immunity, mature ones in adults utilize the complement system to defend against pathogens and capture immune complexes for macrophage elimination. Recent studies demonstrate RBCs' immune activity throughout human life.
Fasting, especially the STIF or 'beego' regimen popular in Asia, which combines water-only fasting with psychological exercises and concludes with refeeding, reshapes the immune landscape by redistributing cells. Further research is necessary to understand how short-term intensive fasting affects the immune response of red blood cells to infectious pathogens, especially SARS-CoV-2.
About the study
The present study obtained informed consent from 31 participants aged 27 to 67 from various Chinese provinces who fasted on water alone for six days, then gradually transitioned to a six-day refeeding, starting with a millet and rice soup and eventually adding noodles, eggs, and vegetables. The Institutional Ethics Review Board at Soochow University (IERB-SU) and the Chinese Clinical Trial Register (CCTR) approved this study.
Blood samples, collected between 7:00 and 9:00 a.m. on specific test days, were subjected to routine analysis using sodium citrate anticoagulant tubes, specifically evaluating the Mean Corpuscular Volume (MCV) and Red Cell Distribution Width (RDW). RBCs were extracted, and their morphology was observed using a Wright-Giemsa stain kit. Concentrations of 2, 3-Diphosphoglycerate (2, 3-DPG) and fetal Hemoglobin (f-Hb) levels in the plasma were determined using specific human Enzyme-Linked Immunosorbent Assay (ELISA) Kits.
For the proteome sequencing in red blood cells, protein extraction involved a sequence of processes using various chemical reagents, centrifugation steps, and heating methods. Following protein extraction, proteins were quantified using Coomassie Brilliant Blue G-250. The extracted proteins underwent high pH RP separation, data-independent acquisition (DIA) quantification, and MSstats differential analysis.
Overrepresentation analysis was conducted on identified Differentially Expressed Proteins (DEPs) using clusterProfiler for both Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms. Gene Set Enrichment Analysis (GSEA) was also performed using the clusterProfiler on preranked gene lists.
For data presentation, statistical data was analyzed with a statistical package for the social science (SPSS) version 22.0. One-way ANOVA and Dunnett's test were employed for multi-group data. P-values from ANOVA were presented, and multiple comparisons were corrected using the Benjamini & Hochberg method. Data is represented as mean ± scanning electron microscope (SEM) and visualized using GraphPad Prism 8.
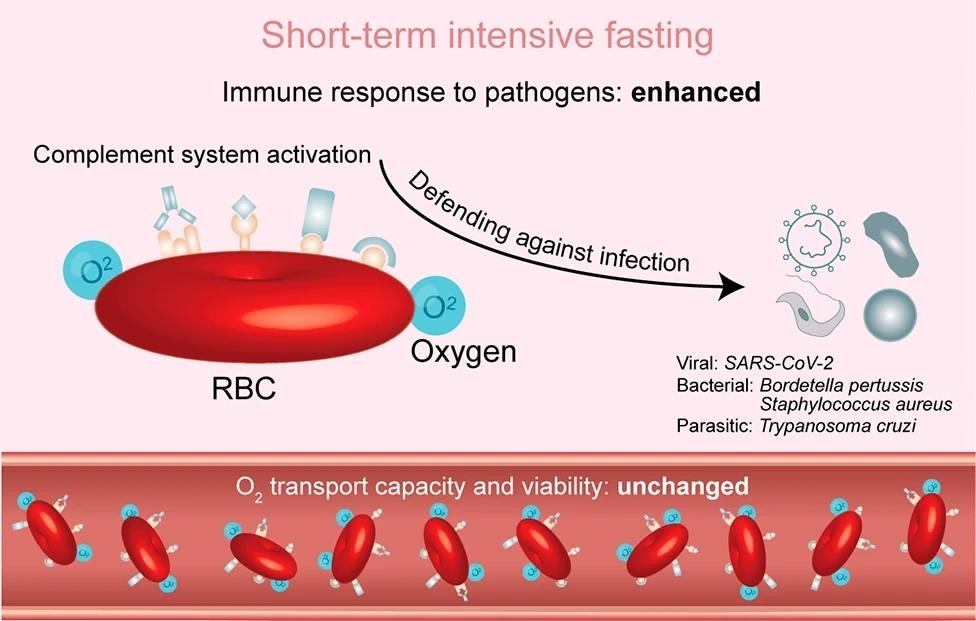
Cartoon illustrating the argument of the immune function of red blood cells by STIF. STIF triggers the activation of the complement system via complement receptors on the membrane of red blood cells to enhance the immune response to various pathogens, particularly SARS-CoV-2. When empowered with upregulated immune function by STIF, red blood cells maintain their oxygen transport capacity and viability
Study results
The present study embarked on an exploration of the impact of STIF on red blood cells. This was conducted on thirty-one subjects who practiced STIF for six days, followed by a six-day refeeding period, a method popular among water-only fasting participants in Asia. The research procured proteomics profiles of red blood cells from a subset of the cohort. The differential analysis revealed significant variations in protein abundance throughout the fasting and refeeding stages.
During the intensive fasting and refeeding process, biological pathways were analyzed, revealing that by the sixth day of fasting, numerous immune response-related pathways were activated, many of which persisted through the sixth day of refeeding. By integrating differential expression analysis with gene set enrichment analysis (GSEA), the researchers established that STIF appears to promote the immune function of red blood cells. This was further solidified by flow cytometric analysis, which indicated an increase in the expression of a protein associated with enhanced immune response in red blood cells.
An in-depth GSEA of the proteome profile in connection with the KEGG database disclosed that STIF significantly impacts how red blood cells respond to pathogens. This became especially intriguing when it was noted that STIF not only activated immune responses against various pathogens but also had a prolonged effect on coronavirus disease (COVID-19).
Further exploration revealed that a significant portion of the enhanced immune response was linked to the complement system, which is vital in mature red blood cells for defense against pathogens. The evidence proposed that STIF activates this system, thereby enhancing the immune function of erythrocytes. Particularly noteworthy was the finding that post-STIF, red blood cells were primed to combat SARS-CoV-2.
However, given that red blood cells primarily transport oxygen, the team questioned whether boosting the immune response might compromise this primary function. Various tests confirmed that the primary function remained intact, with no noticeable changes in the red blood cells' morphology, size, or oxygen-carrying capacity.
Lastly, the viability of the red blood cells during and post-STIF was explored, as overactivation of the complement system can sometimes induce apoptosis. However, results showed no change in the apoptosis levels during STIF, suggesting that the activation of the complement system did not hamper erythrocyte viability. In essence, this study unveiled the multifaceted benefits of STIF on red blood cells, enhancing their immune response without compromising their primary function or viability.
Conclusions
To summarize, in the present study, researchers discovered that STIF amplifies the immune response of human red blood cells against SARS-CoV-2 without compromising their oxygen transport ability, and this enhanced immunity persisted through extended fasting and refeeding periods. Past research showed fasting's impact on leukocyte immune functions, but the effects on human red blood cells were unclear.
The study indicates that red blood cells enhance defenses during leukocyte shortages, especially in their immune reaction to SARS-CoV-2 due to their interactions with the virus in COVID-19 patients; however, further research is essential to comprehend the long-term implications fully.