Avian paramyxovirus type 1 (APMV-1) is a single-strand RNA virus known to cause Newcastle disease, an infectious disease with neurologic, digestive, and respiratory manifestations in birds. In humans, this zoonotic disease usually manifests as mild conjunctivitis and is rarely fatal. In the present case, researchers reported the neurologic disease caused by a pigeon variant of APMV-1 (PPMV-1), primarily spread by pigeons and doves, that resulted in the death of an immunocompromised pediatric patient.
The case
A 2-year-old female with a history of pre-B cell acute lymphoblastic leukemia (ALL), treated with blinatumomab six months ago, presented with nausea and vomiting following upper respiratory symptoms for three weeks. She had undergone the second round of reinduction chemotherapy with 6-mercaptopurine, cytarabine, and cyclophosphamide six weeks ago. There was no history of exposure to illness, pets, or travel. As her condition progressed in the next four days, she developed febrile infection-related epilepsy syndrome (FIRES).
The initial magnetic resonance image (MRI) of the cerebrum was unremarkable. The tests for autoimmune encephalitis were negative. Significant inflammation was observed in the central nervous system (CNS) as indicated by elevated levels of neurotropin (1,752 nmol/L) in the cerebrospinal fluid. Exome sequencing results suggested no genetic abnormalities. No bacterial, fungal, viral, or mycobacterial pathogens were found in culture and polymerase chain reaction (PCR) tests.
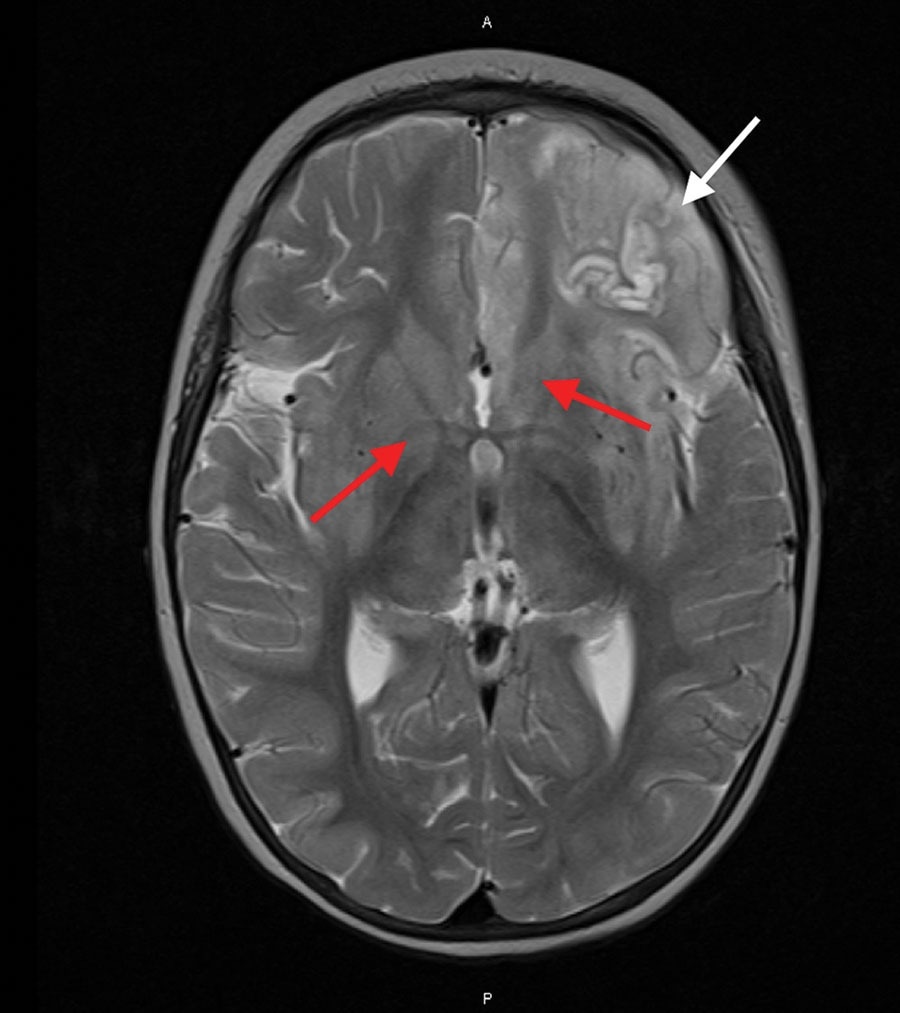
Magnetic resonance imaging of the brain of an immunocompromised child with avian paramyxovirus type 1 infection, Australia. Image, captured 16 days after hospital admission, shows predominantly left frontal and insular T2 signal hyperintensity evolving into laminar necrosis (white arrow) and hyperintensity of deep gray-matter structures (red arrows)
A brain biopsy followed by staining with hematoxylin and eosin of the samples, conducted 20 days post-admission, revealed extensive cortical necrosis with limited subpial sparing. Foamy macrophages and scattered CD3-positive T-cells replaced the cortex, accompanied by gliosis. Viral inclusions, microglial nodules, or viral cytopathic effects were found to be absent. NeuN immunostaining indicated rare remaining small neurons. It is important to note that no viral pathogens were detected in CSF, plasma, or brain tissue, ruling out various viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Negative results were obtained for bacterial and fungal cultures, pan-mycobacterial PCR, and 16S ribosomal ribonucleic acid (RNA) PCR.
The patient’s condition failed to improve despite treatment with immunomodulators, antimicrobials, antiseizure drugs, and being on a ketogenic diet. An MRI taken after two weeks displayed advancing and diffuse inflammatory alterations, characterized by escalating hyperintensity in the left frontal and insular T2 signals, progressing to laminar necrosis. Additionally, T2 hyperintensity was observed in deep gray-matter structures.
The treatment was discontinued, and the patient expired after 27 days of hospitalization. Although post-mortem was not conducted, comprehensive agnostic metagenomic testing and unbiased meta-transcriptomic sequencing were performed parallelly on the biopsied brain tissue. The results revealed the dominant presence of a virulent strain of APMV-1 and low levels of human pegivirus (HPgV) as non-human sequences. Phylogenetic analysis suggested that the virus was from a putative Australian lineage of PPMV-1, belonging to class II, genotype VI, sub-lineage 2.1.1.2.2.
Virus-specific quantitative PCR and immunohistochemistry were used to confirm the APMV-1 infection in the tissue sample. Nucleoprotein clustered cells and pyramidal neurons were observed in the sample tissue, and no staining was identified in the negative controls (juvenile brain and lymphoid tissue).
Discussion
Previous studies have highlighted PPMV-1’s virulence and potential for severe disease as compared to other APMV-1 genotypes. As HPgV is not known to be associated with human disease so far, and no other coinfection was found, the death of the patient was attributed to encephalitis from PPMV-1 infection in the CNS. Given the respiratory symptoms observed in the child, the infection is indicated to have begun in the upper respiratory tract following an inadvertent exposure to infected pigeon feces or fluids.
This is the first report to mark an association between FIRES and avian viruses. As AMPV-1 has been used previously as an oncolytic agent, researchers in the present study recommend careful consideration of the virulence of various strains of this virus and the potential adverse effects of using PPMV-1.
Conclusion
In summary, this case highlights the complex interplay between prior leukemia treatment, infectious triggers, and neurological complications in pediatric patients. It demonstrates the significance of using metagenomics in identifying novel pathogens, diagnosing complex clinical cases, and simplifying the overall workflow. However, the integration of metagenomics in routine diagnostics is limited by its cost and the need for skilled labor. Further research and development addressing these challenges could help increase the accessibility and affordability of this technique, thereby improving patient health outcomes in emerging infectious diseases.