Sustained, cross-reactive immune memory is essential for protection from severe outcomes in the post-coronavirus disease 2019 (COVID-19) pandemic period. Multiple SARS-CoV-2 variants have emerged over time, including the Omicron XBB sublineages. Of late, the Omicron BA.2.86 subvariant has been described, which the World Health Organization has classified as a variant under monitoring due to its hypermutated state.
Further, reports suggest an extensive degree of immune evasion by BA.2.86. The ability of spike-specific T-cells to cross-recognize the BA.2.86 spike is unknown. While studies have demonstrated that infection- or vaccine-induced spike-specific T-cell responses against the ancestral strain are cross-reactive against BA.1, it is necessary to determine whether BA.2.86 mutations hamper its recognition by memory T-cells.
Study: Post-pandemic memory T cell response to SARS-CoV-2 is durable, broadly targeted, and cross-reactive to the hypermutated BA.2.86 variant
The study and findings
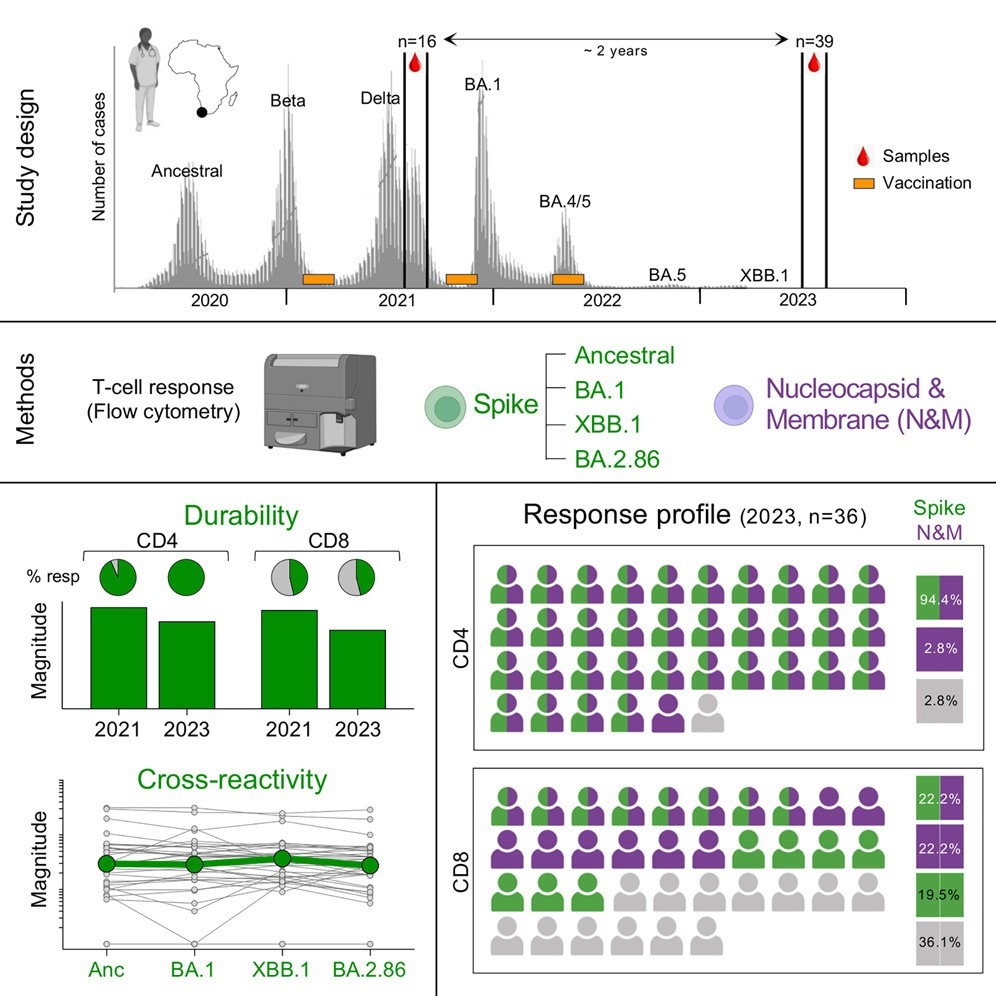
In the present study, researchers assessed the durability of SARS-CoV-2 spike-specific memory T-cell responses. Thirty-nine healthcare workers with a history of vaccination and infection were included. Blood samples were obtained in mid-late 2023. Twenty-two participants received two vaccine doses, 11 received one, and six received three. Besides, 22 participants had a history of infection before the Omicron wave, while all subjects had a breakthrough infection in the Omicron wave.
The researchers measured cytokine production in response to spike peptide pools of the ancestral strain and Omicron BA.1, BA.2.86, and XBB.1 variants. Around 95% of participants mounted a robust cluster of differentiation 4-positive (CD4+) T-cell response to the ancestral spike protein 1.5 years after their last infection.
Spike-specific CD4+ T-cell frequency was not significantly different between Omicron variants. The CD4+ T-cell response was preserved against Omicron variants. By contrast, the proportion of CD8+ T-cells to the ancestral spike protein was markedly lower, and it was consistent among Omicron variants. Some participants lacking a CD8 response to the ancestral spike gained CD8 responses against ≥ one Omicron variant.
Among those with a CD8 response to ancestral spike, most participants had at least half of the CD8 response preserved against Omicron variants, and only some showed a loss of or reduced T-cell reactivity. Further, T-cell responses to nucleocapsid and membrane (N & M) proteins were detected in 35 participants.
Most subjects had CD4 responses to both N & M and spike. By contrast, CD8 responders were evenly distributed among those targeting both (spike and N & M), exclusively N & M and spike only. Furthermore, the team compared the spike- and N & M-specific T-cell frequencies in 15 paired samples to measure T-cell maintenance.
Samples were obtained at two time points: four to six months before the BA.1 wave (T1) and around 1.5 years after that (T2). Between T1 and T2, nine subjects received a booster dose, and all had a breakthrough infection. Spike-specific CD4+ T-cell frequency did not significantly differ between the two time points. All subjects without a CD4 response to N & M mounted a CD4 response by T2.
Only a few participants had detectable CD8+ T-cell responses specific to spike or N & M. The evolution of CD8 responses was variable, with participants showing sustained, lost, or newly acquired responses. The researchers identified four spike-specific memory T-cell subsets – naïve, early differentiated, late differentiated, and effector.
CD4+ T-cells specific to the ancestral spike showed an early differentiated memory profile at T1, which marginally decreased by T2, concomitant with an increase in cells with a late differentiated profile. The memory profile of CD8+ T-cells, defined at T2, was diverse, comprising a median of 20% of effector cells, 20% of late differentiated cells, and 40% of early differentiated cells.
Conclusions
Taken together, the findings indicate robust memory T-cell responses in healthcare workers more than 1.5 years post-Omicron wave. Maintenance of T-cells could be related to recurrent SARS-CoV-2 exposure, expanding T-cell memory pool, or durable responses lasting from before infection and vaccination. Overall, the hybrid immunity results in the accumulation of spike- and non-spike-specific T-cells, with preserved recognition of highly mutated variants.