Everyone’s talking about vaccines, but what about people who have already caught the disease?
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused coronavirus disease 2019 (COVID-19), undoubtedly the worst pandemic in American memory. The viral infection has already surpassed the 1 million death toll and cost the US people billions in unprecedented infrastructure and economic loss. The advent of messenger RNA (mRNA) vaccinations against the disease and the implementation of social distancing restrictions significantly hampered the spread of the disease but did little to treat the millions of patients already burdened with COVID-19 infections.
Antiviral drugs have shown great promise in treating individuals with viral ailments, most notably in the case of antiretroviral therapy against human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS). Historically, oseltamivir, an antiviral used against the 2009 influenza A (H1N1) pandemic, is estimated to have saved 63% of hospitalizations. Paxlovid, a Pfizer-developed antiviral, has the potential for even greater years of lives lost (YLL) and economic savings for the US. The drug, a combination of nirmatrelvir and ritonavir, two antivirals with clinically proven anti-COVID-19 efficacy, received Food and Drug Administration (FDA) Emergency Use Authorization on December 22, 2021.
Research on the benefits of Paxlovid has hitherto revealed that “treating symptomatic COVID-19 patients with Paxlovid reduces hospitalization risks by an estimated 0.59 for adults 18–49 years of age, 0.40 for adults 50–64 years of age, and 0.53 for adults >64 years of age.” Most encouragingly, Paxlovid has proven effective against Omicron subvariants, responsible for most of the current global COVID-19 burden, and the most aggressive clade of SARS-COV-2 yet. However, predictions addressing the true benefits of the drug remain lacking.
About the study
In the present study, researchers used data from a cohort of more than 2,200 COVID-19 patients to analyze the population-level benefits of Paxlovid utilization. They then built within-host viral replication models to simulate 24 separate COVID-19 transmission scenarios.
Data for the study comprised individual patient viral loads from a Paxlovid clinical trial, including 1,120 cases receiving Paxlovid and 1,126 controls receiving a placebo. The data was used to reveal 1. infection rates of susceptible cells (b), 2. the rate at which infected cells die (c), 3. the virus production rate (p), and the efficacy of Paxlovid at suppressing viral replication (). A stochastic approximation expectation-maximization algorithm was used to improve simulation accuracy.
“On the basis of previous studies, we assumed that a person’s infectiousness is logarithmically related to their viral titer (Appendix). In this transmission model, we assumed that the daily infectiousness of a case-patient depends on whether they received treatment and, if so, the time at which treatment was initiated after symptom onset.”
To further improve the real-world application of the models, the simulations were tuned to 9,961 persons living in 5,000 households whose sociodemographic data was acquired from the National Household Travel Survey (2017). Results from Paxlovid versus no intervention were used to compute YLL averted metrics and monetary savings.
Study findings
Findings from the study revealed that the average rate of viral infections of susceptible cells is 3.92 x 10−6 mL/copies/day, the clearance rates were 0.62 per day, and the production rates were 3.19 mL/copies/day. Impressively, Paxlovid treatment was estimated to suppress 99.37% of viral replication.
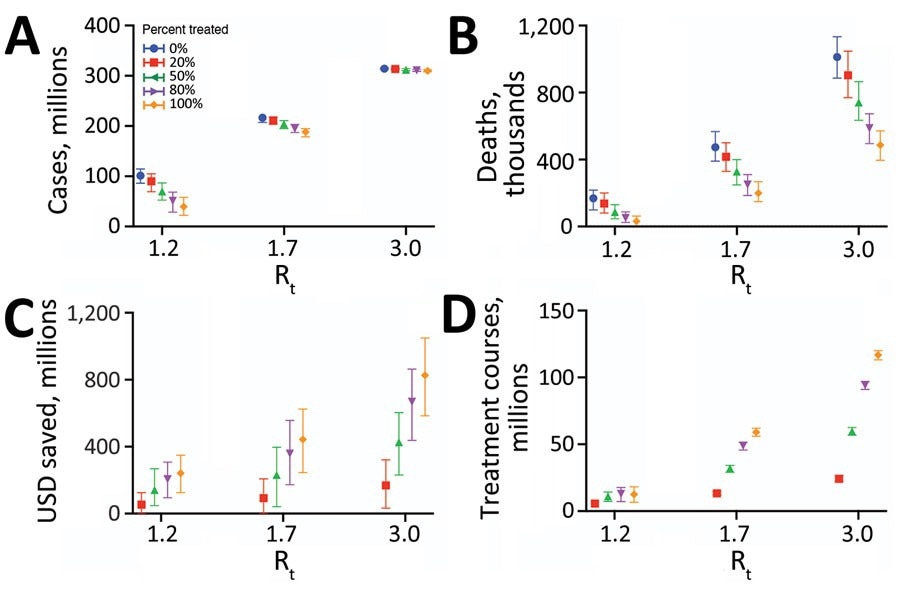
Projected health and economic impacts of a large-scale campaign using Paxlovid to treat COVID-19 over 300 days in the United States, across a range of transmission and treatment scenarios. Points and error bars correspond to means and 95% CI in number of infections in millions (A), number of deaths in millions (B), net monetary benefit in billions USD assuming a treatment course cost of US $530 and willingness to pay per year of life lost averted of US $100,000 (C), and number of courses of Paxlovid administered in millions (D). Each graph provides results for 3 Rt and 5 different treatment scenarios: 0% (blue), 20% (red), 50% (green), 80% (purple), or 100% (orange) of symptomatic cases started a 5-day course of Paxlovid within 3 days of symptom onset. Distributions are based on 100 stochastic simulations for each scenario. The results are scaled assuming a US population of 328.2 million (21). Rt, effective reproduction number; USD, US dollars.
Of the 24 transmission scenarios simulated, the low- (reproduction number = 1.2) and high-transmission (number = 3) ones revealed YLL aversions of between 0.28 and 0.85 million patients and economic savings of between US $56.95 billion to US $170.17 billion if even only 20% of COVID-19 patients were treated with the antiviral drug.
When teasing apart the direct benefits (therapeutic) and indirect (transmission-reducing) effects of Paxlovid, researchers found that over 300 days, early Paxlovid interventions could reduce COVID-19-related mortality by 16,470 cases and avert 140,000 hospitalizations. Encouragingly, the indirect benefits matched the direct ones and were also predicted to reduce infection rates substantially by 10.57 million or more.
“We would expect mass treatment campaigns to have even greater health and economic effects in countries that have adopted zero-COVID strategies and thus have lower levels of population-level immunity than the United States.”
Conclusions
The present study highlights the benefits to human lives and monetary savings that antivirals like Paxlovid can provide in the fight against COVID-19. Analyses and simulation modeling of data from over 2,200 patients revealed that treating only 20% of the affected population with the drug could save 0.85 million patients and prevent 140,000 hospitalizations per day, curbing economic burden by US $170.17 billion or more.
“Drugs like Paxlovid could profoundly reduce the severity of COVID-19 and enable a global transition to manageable coexistence with the virus. However, providing equitable and effective global access to SARS-CoV-2 antiviral drugs would require both ample supplies and broad-reaching test-and-treat programs.”