Diabetes, a growing public health issue, continues to surge despite extensive research and healthcare efforts. It leads to various forms of diabetic myopathy, irrespective of its type, causing a decline in skeletal muscle mass and function. This decline not only worsens obesity and hyperglycemia but also affects locomotion, energy metabolism, and glucose regulation, further deteriorating muscle structure and function. Additionally, diabetes impairs muscle regeneration, potentially worsening conditions like ischemia and foot ulcers by promoting fibrosis and hindering myofiber recovery. Further research is needed to better understand and develop targeted interventions for the complex mechanisms underlying impaired muscle regeneration in diabetes.
Skeletal muscle abnormalities in diabetes
Diabetes, alongside its comorbidities like obesity, hypertension, and dyslipidemia, significantly affects skeletal muscle structure, function, and metabolism. The complex nature of diabetes complicates the identification of effective therapeutic targets. Other contributing factors include aging, inactivity, and poor nutrition. Key observed abnormalities in diabetic patients include reduced muscle mass and strength, abnormal lipid deposition, fiber atrophy, and altered myokine secretion, contributing to decreased functional capacity and quality of life.
Diabetes not only leads to muscle degeneration but also impairs the muscle's ability to regenerate, complicating injuries such as ischemia and foot ulcers. The regeneration process, involving both muscle stem cells (MuSCs) and non-MuSCs, is hampered, as indicated by excessive fibrosis and delayed myofiber maturation.
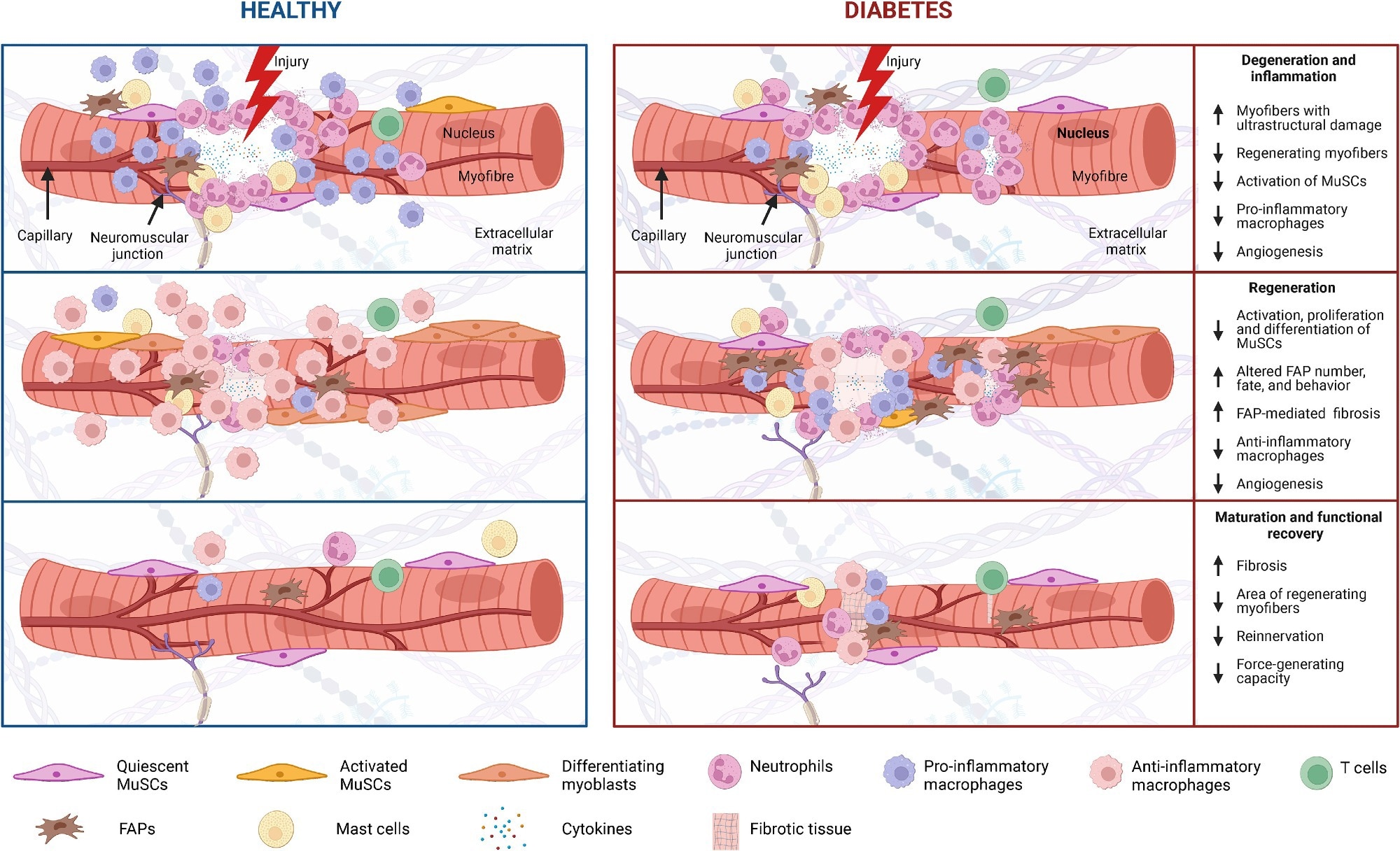
Skeletal muscle regeneration in diabetes Diabetes and its associated complications, including obesity and hyperglycemia, impact multiple cell populations (MuSCs, neutrophils, macrophages, T cells, FAPs, and mast cells) that play a vital role in the process of muscle regeneration (i.e., degeneration and inflammation, regeneration, and maturation and functional recovery).
Degeneration and inflammation
Muscle injuries trigger necrosis and inflammation, marked by fiber breakdown and protein leakage into the serum. The process, essential for tissue repair, draws in immune cells like neutrophils and macrophages. Diabetes compounds this degeneration, amplifying damage, and hampering regeneration, highlighting the metabolic impact on muscle recovery.
Regeneration process
Diabetes negatively impacts the muscle regeneration process, notably affecting the activation, proliferation, and differentiation of MuSCs and the roles of fibro-adipogenic progenitors (FAPs). Treatments like metformin offer some hope by potentially modifying FAP activity. However, delayed regeneration in diabetic models underlines the urgent need for deeper insights into how diabetes disrupts muscle repair mechanisms.
Challenges in muscle recovery
Efficient muscle regeneration requires not only the formation of new myofibers but also the reconstitution of the extracellular matrix, vascular network, and innervation. Diabetes and obesity complicate this process, showing delayed functional recovery, increased collagen accumulation, and impaired neuromuscular junction adaptations.
Diabetic impacts on muscle fiber and insulin signaling
Diabetes shifts muscle fiber composition towards type II fibers, which are more prone to damage and impair regeneration. Insulin resistance disrupts muscle cell growth pathways, while hyperinsulinemia and lipotoxicity inhibit crucial recovery processes like autophagy and protein metabolism. These changes suggest that targeting fiber-type transitions and improving insulin signaling could enhance muscle regeneration in diabetes.
Diabetic challenges in muscle regeneration signaling
Diabetes triggers elevated pro-inflammatory cytokines and oxidative stress, disrupting muscle repair by inhibiting growth pathways and promoting protein breakdown. Concurrently, increased myostatin levels and NOTCH and WNT signaling alterations impair muscle cell proliferation and differentiation. Moreover, the compromised Adenosine Monophosphate-Activated Protein Kinase (AMPK) signaling pathway further hinders MuSC function and regeneration, highlighting complex challenges in diabetic muscle repair.
Disentangling diabetes and comorbidity effects on muscle regeneration
Diabetes significantly impairs muscle regeneration, but pinpointing whether diabetes itself or related comorbidities such as obesity and sarcopenia are responsible remains challenging. Muscle health is influenced by a number of factors, including genetics, diet, and physical activity, complicating the isolation of diabetes' direct effects. Studies often struggle to establish control groups that adequately account for these variables, leading to ambiguity about the specific impacts of diabetes versus other conditions. For instance, research using obese diabetic mice versus lean controls has difficulty distinguishing whether observed effects are due to obesity or diabetes itself.
Challenges in research models and therapeutic approaches
There is no definitive animal model for studying diabetes' impact on muscle regeneration, complicating the translation of findings to humans. Treatments for muscle regeneration in diabetes are varied, spanning from exercise and dietary supplements to advanced cell therapies, yet their effectiveness often falls short in addressing muscle fibrosis. Despite the promise shown by certain therapies in improving muscle health in diabetes, rigorous clinical trials are needed to assess their true efficacy in muscle regeneration, specifically within diabetic populations.
Future directions in muscle regeneration research
Addressing these gaps requires a multifaceted approach. Research must refine its models and control groups to isolate the effects of diabetes from those of comorbidities and lifestyle factors. Advanced genetic and omics technologies offer new avenues to uncover the intricate mechanisms at play in diabetic muscle regeneration. Furthermore, integrating therapies such as exercise, dietary interventions, and possibly cell therapies may hold the key to enhancing muscle repair in diabetic patients. However, more research is essential to navigate the complexities of muscle regeneration in diabetes and develop effective treatments.