The germ-disease theory, created in the nineteenth century, is a critical medical paradigm claiming that pathogenic microbes cause infectious diseases. However, this explanation is microorganism-centric, failing to explain varying disease severity and symptomatic profiles among individuals. While the microorganism is necessary to produce illness, it cannot solely determine the infection outcome. As a result, the theory must consider the host's status fluctuations, which considerably impact infection outcomes.
Recent research questions the theory, giving rise to the full-blown host theory, which holds that infectious illnesses are caused by inherited or acquired immunodeficiencies in the host. According to this hypothesis, viruses are passive environmental triggers, and disease development results from pre-existing host immunodeficiencies. These inadequacies can be covert or overt, depending on diagnostic procedures used to identify critical diseases.
About the review
In the present review, researchers investigated the relative relevance of pathogenic microbes and hosts in determining infection outcomes, revealing a non-centric perspective that acknowledges essential roles for both the pathogenic microorganism and the host in defining infection outcomes.
Pathogens and virulence
Pathogens are germs that can cause illness among immunocompetent and healthy hosts; nevertheless, asymptomatic carriership occurs when pathogens infect or colonize hosts without causing symptoms. Examples include Helicobacter pylori and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which can cause asymptomatic infections in 40% to 80% of cases. Mycobacterium tuberculosis is the most prominent example of pathogens producing asymptomatic carriership, with one-fourth of the worldwide human population latently infected but exhibiting no symptoms of illness.
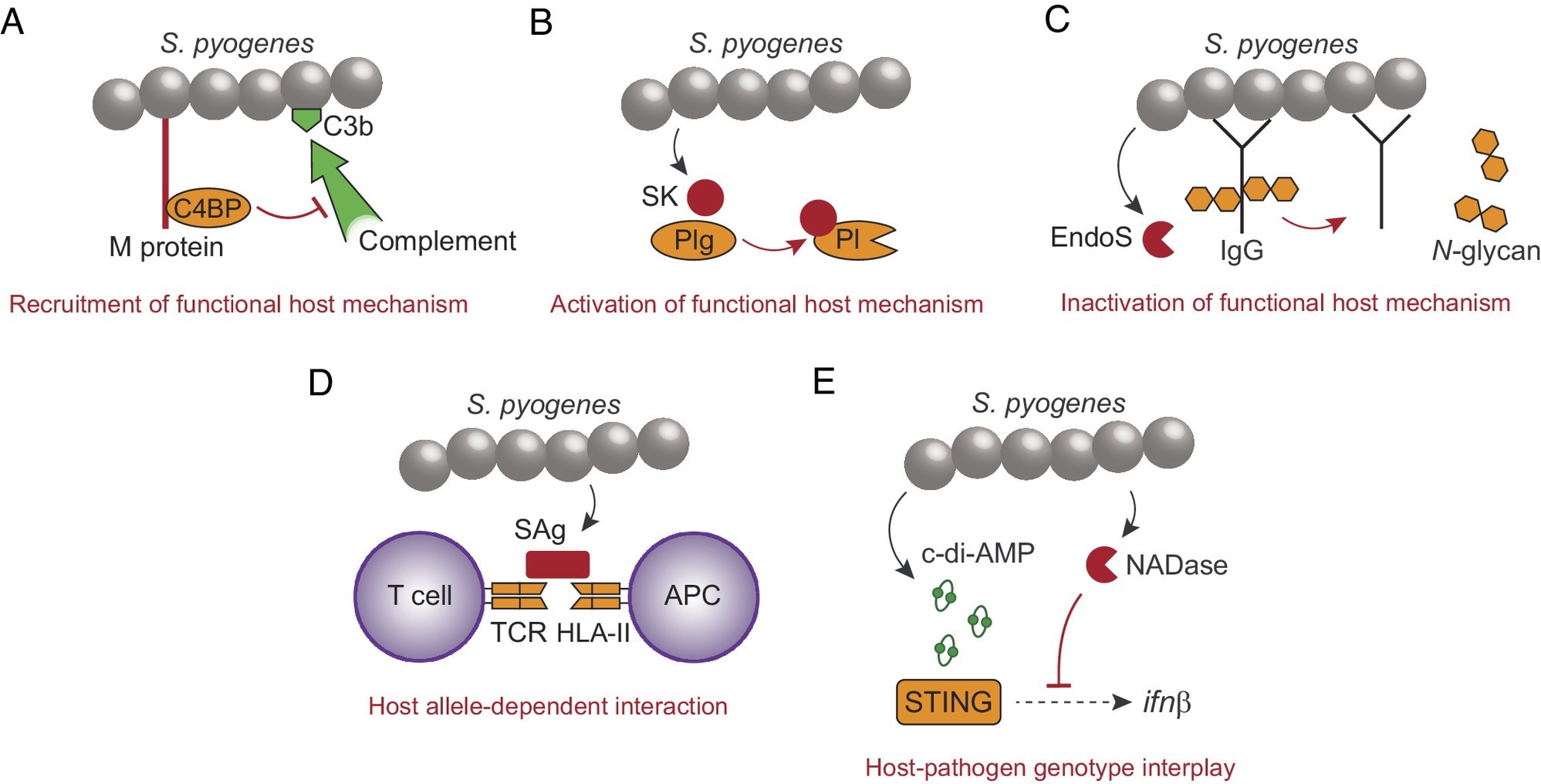 Examples demonstrating the evolved ability of S. pyogenes to promote disease in immunocompetent individuals. (A) The surface M protein recruits human C4BP to inhibit complement opsonization (C3b) of the bacterial surface. (B) Secreted streptokinase (SK) binds to human Plg, which causes a conformational change of Plg into a plasmin (Pl) active state. (C) The secreted endoglycosidase EndoS inactivates effector functions of IgG by cleaving off N-glycans from the Fc-region. (D) Secreted superantigen (SAg) causes antigen-independent T cell activation by cross-linking the TCR with HLA-II on antigen presenting cells (APC). SAgs have different affinity for different fully functional HLA-II haplotypes. (E) The STING responds to S. pyogenes-derived c-di-AMP to induce transcription of the interferon β gene, which is inhibited by the enzymatic activity of bacterial NADase. Human STING and S. pyogenes NADase exhibit polymorphisms affecting their relative ability to respond to c-di-AMP and to suppress interferon transcription, respectively.
Examples demonstrating the evolved ability of S. pyogenes to promote disease in immunocompetent individuals. (A) The surface M protein recruits human C4BP to inhibit complement opsonization (C3b) of the bacterial surface. (B) Secreted streptokinase (SK) binds to human Plg, which causes a conformational change of Plg into a plasmin (Pl) active state. (C) The secreted endoglycosidase EndoS inactivates effector functions of IgG by cleaving off N-glycans from the Fc-region. (D) Secreted superantigen (SAg) causes antigen-independent T cell activation by cross-linking the TCR with HLA-II on antigen presenting cells (APC). SAgs have different affinity for different fully functional HLA-II haplotypes. (E) The STING responds to S. pyogenes-derived c-di-AMP to induce transcription of the interferon β gene, which is inhibited by the enzymatic activity of bacterial NADase. Human STING and S. pyogenes NADase exhibit polymorphisms affecting their relative ability to respond to c-di-AMP and to suppress interferon transcription, respectively.
In contrast, opportunistic microbes cannot cause illness in usual settings but can be pathogenic in the case of compromised host steady-state conditions. For example, Clostridium difficile, a spore-forming bacteria, can cause symptomatic illness when treated with antibiotic medications, which reduces the microbiota and allows Clostridium difficile germination to develop a footing. Commensal Candidal fungi can also cause illness in regular settings, but only in the case of immunosuppression, as observed in individuals with HIV-associated immunodeficiency syndrome (AIDS).
Virulence, a pathogen's capacity to generate illness symptoms or pathology, is vital to infection outcomes. According to evolutionary theory, a pathogen's Darwinian fitness is often maximized at an intermediate degree of virulence, resulting in fitness costs and benefits. This idea gains support by controlled laboratory tests and epidemiological research on human illnesses such as AIDS.
Host-pathogenic microbe interactions and genetic variability
Streptococcus pyogenes, a bacterium often present in humans, can cause asymptomatic carriership and symptomatic illnesses such as septic shock and necrotizing infections in soft tissues. Streptococcus pyogenes' surface M proteins are polymorphic virulence factors that impart phagocytic resistance and prevent complement opsonization, recruiting the serum C4b-binding protein (C4BP) and enabling, allowing rapid bacterial proliferation in blood.
A secreted Streptococcus pyogenes protein, streptokinase, preferentially binds with and stimulates human plasminogen (Plg), promoting proteolytic actions and bacterial spread. Transgenic mice infection revealed the importance of this relationship in virulence, revealing that Streptococcus pyogenes activates fibrinolytic pathways to cause illness.
The association of bacterial virulence characteristics with the genetic diversity of the host is critical. Superantigens (SAgs) of streptococcal species form cross-links with T-cell receptors (TCRs) to human major histocompatibility complex (MHC) class II molecules, causing antigen-independent T-lymphocyte activation. Fully functional human leukocyte antigen II (HLA-II) variations considerably affect infection outcomes. Type I interferon (IFN) signaling protects Streptococcus pyogenes-infected mice against host-damaging inflammation.
Uncommon mutations that profoundly impact infection outcomes cause Casanova's inborn immunity defects. More frequent alleles with lesser effects play a primary role in the phenotypic variance in infection outcomes, maintained by balanced selection. In addition to host genetic diversity, pathogens exhibit enormous genetic variation, and the combination of host and pathogen genotypes considerably impacts disease severity.
Based on the review findings, microbes have evolved to flourish in extreme conditions such as polar ice, scorching vents, sulfur-acidic lakes, and immunocompetent hosts. Pathogens have developed methods to avoid host defenses, allowing them to infect and produce clinical illness in immunocompetent humans. The review stresses that the host and the pathogen's genetic diversity can impact infection outcomes, implying that both species play active roles. The researchers' proposed full-fledged host theory is disproven in favor of a non-centric approach, recognizing crucial roles for the causal bacterium and the host in determining infection outcome.