Scientists at the Georgia Institute of Technology and the University of Wisconsin, USA, have developed a broad-spectrum vaccine that can protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants as well as bat sarbecoviruses.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently emerged beta-coronavirus responsible for the devastating coronavirus disease 2019 (COVID-19) pandemic.
SARS-CoV-2 contains spike glycoprotein on its surface, which interacts with the host cell membrane receptor angiotensin-converting enzyme 2 (ACE2) to facilitate viral entry. This makes spike protein a crucial target for vaccine development.
Several vaccines have been developed throughout the pandemic to restrict the transmission of SARS-CoV-2 and its highly mutated variants. Pfizer-BioNTech’s bivalent vaccine is one of the updated variant-specific formulations with high protective efficacy against a range of SARS-CoV-2 variants.
Besides the emergence of a wide range of SARS-CoV-2 variants, an extensive reservoir of ACE2-binding sarbecoviruses has been identified in bats, which highlights the need for developing pan-sarbecovirus and pan-betacoronavirus vaccines.
In this study, scientists have developed bivalent and trivalent vaccine formulations using a spike protein nanoparticle platform and validated these formulations in hamsters.
Vaccine design
The scientists had previously developed a nanoparticle-based vaccine displaying multiple copies of SARS-CoV-2 spike protein, which showed high protective efficacy against SARS-CoV-2 in hamsters. In the current study, they used this spike protein nanoparticle platform to develop a cocktail vaccine against human ACE2-binding clade 1 sarbecoviruses with pandemic potential.
They developed a panel of nanoparticle platforms, each displaying a single spike protein from various sarbecoviruses (ancestral SARS-CoV-2 spike protein; four omicron variants BA.1, BA.5, BA.2.75.2, XBB; SARS-CoV-1; and bat CoV SHC014). They determined the immunogenicity of these formulations in hamsters.
They analyzed the antigenic landscape and selected one bivalent (two prominent spike proteins) formulation and two trivalent (three prominent spike proteins) formulations to characterize their neutralizing antibody responses and protective efficacy in hamsters challenged with SARS-CoV-2 omicron variants (XBB.1 and BA.5) as well as bat coronaviruses (SHC014 and WIV1).
Validation of vaccine formulations
The scientists immunized hamsters with the bivalent or the trivalent vaccine formulations adjuvanted with Alhydrogel. They also immunized a separate set of hamsters with the Pfizer-BioNTech bivalent vaccine as a positive control.
The analysis of humoral immune responses revealed that all three formulations could effectively induce neutralizing antibody titers against the ancestral SARS-CoV-2 and the omicron variant BA.5.
Two trivalent vaccines containing an additional omicron strain induced robust neutralizing titers against the latest omicron variant XBB.1. However, the bivalent vaccine showed reduced neutralizing efficacy against the XBB.1 variant.
Regarding bat coronaviruses, all three formulations showed equivalent neutralizing efficacies against SHC014 and WIV1. In contrast, the Pfizer-BioNTech bivalent vaccine failed to induce detectable neutralizing antibody titers against any of the tested coronaviruses.
Two doses of Pfizer vaccine containing 30 micrograms of spike protein are typically used for immunization in humans. However, only a single immunization with 10 micrograms of spike protein was applied in hamsters. This might explain the absence of neutralizing titers in hamsters immunized with the Pfizer vaccine.
The scientists further tested the protective efficacy of three vaccine formulations in hamsters infected with omicron variants BA.5 and XBB.1 six weeks after immunization. They tested viral titers in the lungs three days after the viral challenge.
The findings revealed that all three formulations are able to provide complete protection against both BA.5 and XXB.1 variants. No detectable viral titers were observed in the lungs of immunized hamsters.
Despite inducing significantly lower neutralizing titers against XBB.1, the bivalent formulation fully protected the hamsters against XBB.1. However, a single immunization with the Pfizer vaccine failed to provide complete protection against BA.5 and XBB.1.
The scientists further explored the protective efficacy of their trivalent formulation against bat coronaviruses. They found that the immunized hamsters have no detectable viral titers in the lungs, indicating complete protection.
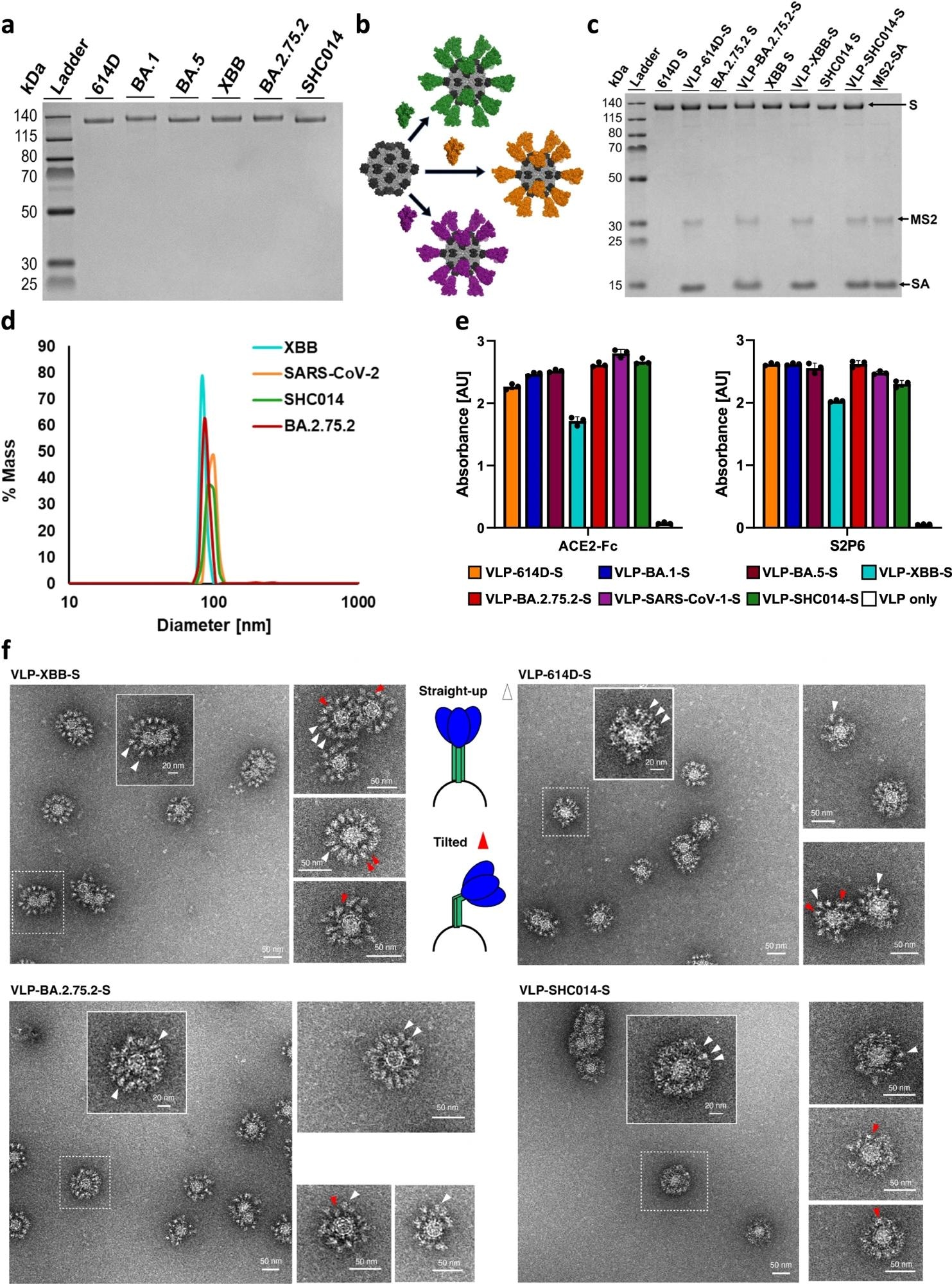 a SDS-PAGE characterization of biotinylated S proteins. The unprocessed gel is shown in Supplementary Fig. 3. This gel was run twice from the same preparation for each sample with similar results. b Schematic of the attachment of various biotinylated S proteins to MS2-SA. (MS2: light gray, PDB 2MS2; SA: dark gray, PDB 3RY2; S: green/orange/purple, PDB 6VSB) (c) SDS-PAGE gel of S and VLP-S for 614D, BA.2.75.2, XBB, and SHC014. Each VLP-S has been boiled to disrupt the streptavidin-biotin conjugation. The unprocessed gel is shown in Supplementary Fig. 3. This gel was run twice from the same preparation for each sample with similar results. d Characterization of VLP-614D-S (orange), VLP-SHC014-S (green), VLP-BA.2.75.2-S (red), and VLP-XBB-S (cyan) by dynamic light scattering. e Characterization of the binding of ACE2-Fc and S2P6 antibody to all VLP-S. (mean ± SD, n = 3: one independent assay with three technical replicates). Bar color identifies each VLP-S sample (VLP-614D-S: orange; VLP-BA.1-S: dark blue; VLP-BA.5-S: brown; VLP-XBB-S: cyan; VLP-BA.2.75.2: red; VLP-SARS-CoV-1-S: purple; VLP-SHC014-S: green; VLP only: white). f Characterization of VLP-XBB-S, VLP-614D-S, VLP-BA.2.75.2-S, and VLP-SHC014-S by negative stain transmission electron microscopy. Arrowheads ▲ indicate S proteins on the VLP surface, with white arrowheads indicating straight-up spike proteins and red arrowheads indicating tilted spike proteins. At least 70 images were collected and analyzed from one VLP-S preparation for each sample with similar results.
a SDS-PAGE characterization of biotinylated S proteins. The unprocessed gel is shown in Supplementary Fig. 3. This gel was run twice from the same preparation for each sample with similar results. b Schematic of the attachment of various biotinylated S proteins to MS2-SA. (MS2: light gray, PDB 2MS2; SA: dark gray, PDB 3RY2; S: green/orange/purple, PDB 6VSB) (c) SDS-PAGE gel of S and VLP-S for 614D, BA.2.75.2, XBB, and SHC014. Each VLP-S has been boiled to disrupt the streptavidin-biotin conjugation. The unprocessed gel is shown in Supplementary Fig. 3. This gel was run twice from the same preparation for each sample with similar results. d Characterization of VLP-614D-S (orange), VLP-SHC014-S (green), VLP-BA.2.75.2-S (red), and VLP-XBB-S (cyan) by dynamic light scattering. e Characterization of the binding of ACE2-Fc and S2P6 antibody to all VLP-S. (mean ± SD, n = 3: one independent assay with three technical replicates). Bar color identifies each VLP-S sample (VLP-614D-S: orange; VLP-BA.1-S: dark blue; VLP-BA.5-S: brown; VLP-XBB-S: cyan; VLP-BA.2.75.2: red; VLP-SARS-CoV-1-S: purple; VLP-SHC014-S: green; VLP only: white). f Characterization of VLP-XBB-S, VLP-614D-S, VLP-BA.2.75.2-S, and VLP-SHC014-S by negative stain transmission electron microscopy. Arrowheads ▲ indicate S proteins on the VLP surface, with white arrowheads indicating straight-up spike proteins and red arrowheads indicating tilted spike proteins. At least 70 images were collected and analyzed from one VLP-S preparation for each sample with similar results.
Study significance
The trivalent spike protein nanoparticle formulation developed in the study shows high efficacy in inducing a broadly neutralizing antibody response against SARS-CoV-1- and SARS-CoV-2-related sarbecoviruses.
Ravi Kane, one of the paper's corresponding authors, stated, “This vaccine may protect not just against the current strain circulating that year but also future variants.”