Norwood, MA – Instron is excited to announce the release of the next generation Autoinjector Testing System for full functionality testing of pen and autoinjectors to ISO 11608. Developed in close partnership with pharmaceutical companies and CDMOs, this system measures a variety of essential performance requirements, including cap removal, dose accuracy, activation force, injection time, needle depth, and needle guard lockout.

Image Credit: Instron
The ability to perform a complete sequence of tests on one system, rather than multiple systems, simplifies testing, data consolidation, and the validation process, and streamlines tech transfer to production sites.
The addition of simple autoinjector test methods to Instron’s Bluehill Universal software empowers users to easily develop and change test methods. The user simply selects which tests (cap removal, injection, and/or needle guard lock out check or defeat force) are to be run and sets the desired parameters. The system then automatically runs through the test sequence and provides results including cap removal and activation forces, injection time, needle depth measurements at the start and end of injection, click detection, delivered volume, and safety check results.
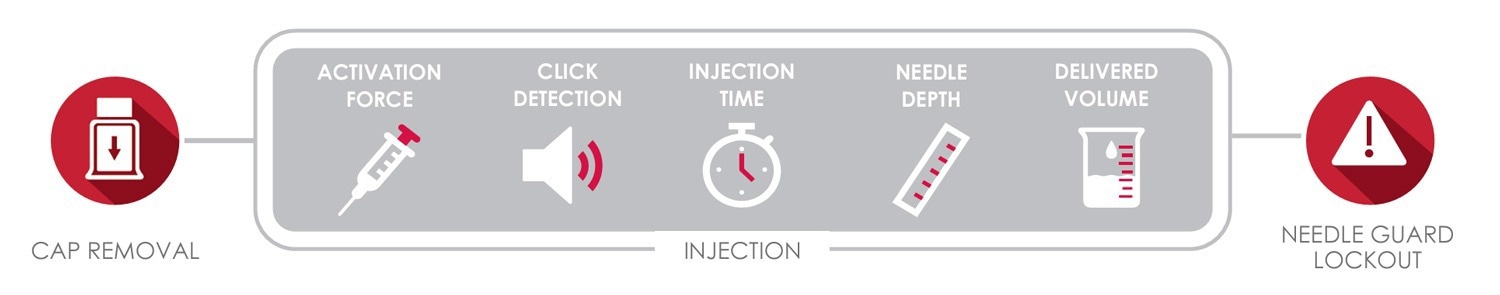
Image Credit: Instron
“Throughout the development of this system, the user interface and experience has been a major focus,” explains Theresa Smith, Product Manager at Instron. “Our customers in development settings require flexibility as they work with new devices and combined products. With this system, they can easily and quickly change setups to test different devices or perform challenge testing. At the same time, our Bluehill software offers the simplicity and traceability that is needed in production settings. It can be a challenge to design for those very different functions, so this system’s adaptability is really exciting.”
Bluehill Universal for autoinjectors also improves compliance with Good Manufacturing Practices (GMP) by integrating system suitability testing (SST) into its workflow. SST prompts users to perform daily verification checks on the load cell, machine vision camera, and scale. The system only allows device testing to proceed once these checks have been successfully completed. SST details are recorded in the Traceability module’s audit log, offering a robust and searchable record.
To simplify tech transfer, Instron’s Bluehill Central lab management software can be added to streamline management and transfer of both device and SST test methods between testing systems in the same lab and other sites around the world.
Instron also offers services, including on-site calibrations and IQOQ services, to help testing labs accelerate their validation process.
Autoinjector Testing System | Semi-Automated System for Testing Pen and Autoinjectors to ISO 11608
About Instron
Instron is a leading global manufacturer of testing equipment for the material and structural testing markets used to evaluate materials ranging from biological tissue to advanced high-strength alloys. Instron systems perform a variety of tests such as compression, cyclic, fatigue, impact, multi-axis, rheology, tensile, and torsion. Instron is a wholly owned subsidiary of Illinois Tool Works Inc. For more information, visit www.instron.com.
