Scientists at the University of California, USA, have developed a precision-guided sterile insect technique to eliminate the primary African malaria vector, Anopheles gambiae mosquitoes, and subsequently reduce malaria transmission.
Malaria is a life-threatening parasitic disease that spreads to humans through mosquito biting. According to the 2022 World Health Organization (WHO) factsheet, there were an estimated 249 million malaria cases and 608,000 malaria deaths across 85 countries.
The widespread distribution of the malaria vaccine has effectively prevented the worst disease outcomes in recent years. However, disease eradication in the most highly infected regions requires completely eliminating the primary African malaria mosquito vector Anopheles gambiae.
Conventional methods, including insecticide-based technologies and environmental controls, are becoming less effective because of the faster emergence of drug-resistant mosquito populations. This highlights the need for developing novel control measures to break the chain of disease transmission.
The sterile insect technique (SIT) is a promising control measure that acts through the mass releases of infertile males, which naturally locate, copulate with, and sterilize their monandrous (having only one male sexual partner) female mates.
The conventional sterilization processes include the transfer of radio-sterilized defective sperm and copulation with a sperm-less male. The development of a scalable genetic SIT system in mosquitoes requires a combination of precise male sterilization and female elimination systems. This is called precision-guided SIT.
In this study, scientists have developed a precision-guided SIT in Anopheles gambiae for inducible, programmed male sterilization and female elimination, which can be used on a large scale in SIT operations.
Development of precision-guided sterile insect technique
The scientists developed a multi-guide RNA (gRNA) line targeting the well-characterized female-essential locus doublesexF (dsxF) and male-fertility genes Zero population growth (zpg) and β2-tubulin (β2).
Using a binary CRISPR strategy, they crossed this gRNA line to a Cas9 transgenic line and observed significant but incomplete female androgenization (development of secondary male sextual characteristics) in the hybrid progeny. This could be due to dsxF targeting, as mentioned by the scientists.
They further improved female elimination by crossing the gRNA line to the Ifegenia Genetic Sexing System line, establishing double homozygous gRNA-expressing lines. The Ifegenia Genetic Sexing System line targets the female-essential gene femaleless (fle) to enable an egg-based distribution modality.
By crossing these two lines to Cas9, they confirmed the presence of mutations within each gene in the hybrid progeny.
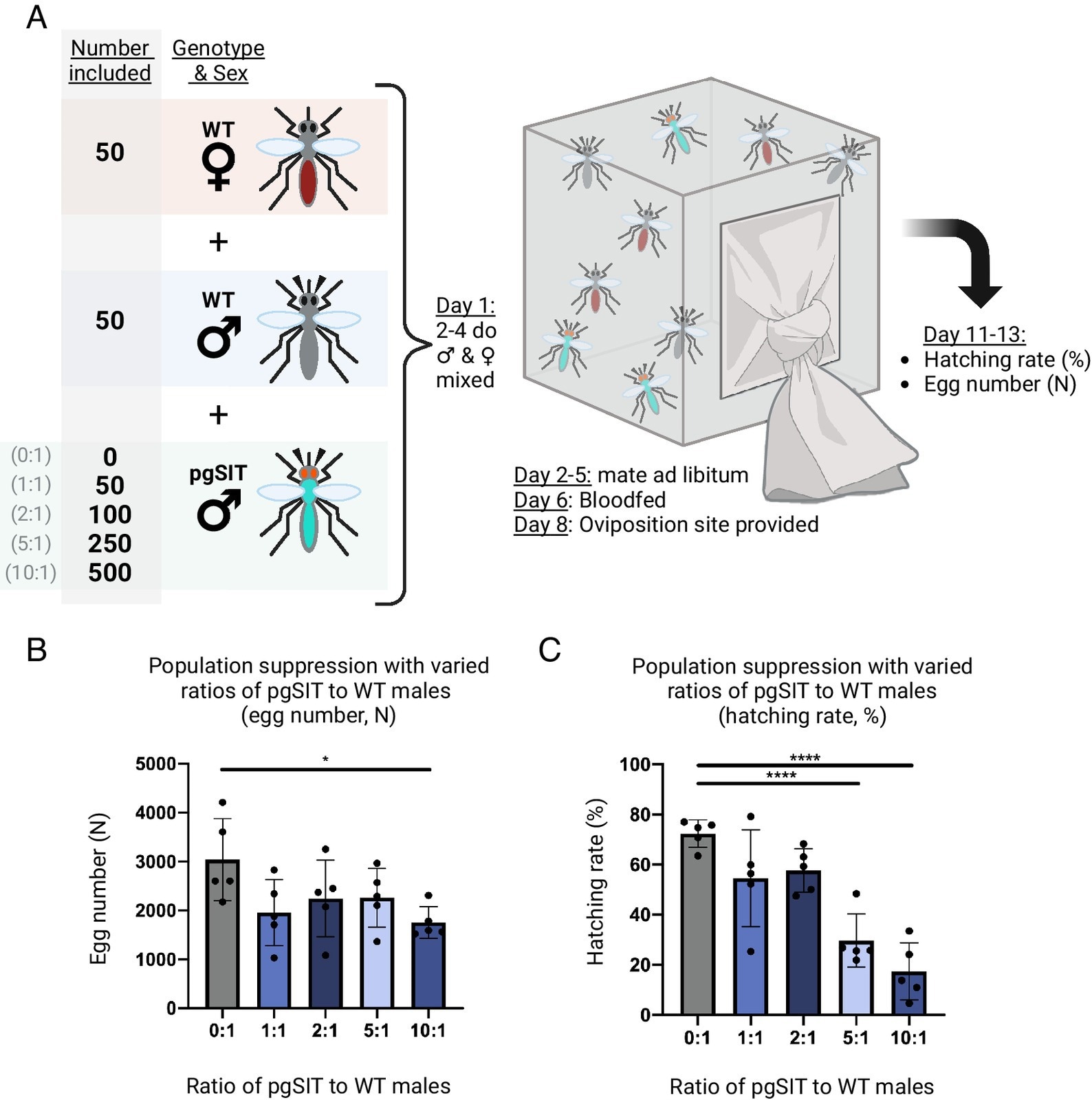 Population suppression following the release of pgSIT males at different ratios to wild type. (A) Test suppression cages were established with 50 wild-type males, 50 wild-type virgin females, and either 0, 50,100, 250, or 500 pgSITD15 males (for the 0:1, 1:1, 2:1, 5:1, and 10:1 pgSIT:wild-type male ratios respectively). After mating and blood feeding, the hatching rate was calculated for each cage. (B) The egg counts from population suppression assay cages. Groups 0:1 and 10:1 are significantly different (P < 0.05, Dunnett’s multiple comparisons). Mean and SD shown. (C) Population suppression as measured by the hatching rate (%) from cages suppressed by different ratios of pgSIT males to wild type males. The hatching rate is reported as the percent of eggs that hatched (n% = n 1-d-old larvae/n eggs laid). The 0:1 control group differs significantly with both the 5:1 (P < 0.001) and 10:1 (P < 0.001) groups (one-way ANOVA, Dunnett’s multiple comparisons test). Mean and SD shown. Created with Biorender.com.
Population suppression following the release of pgSIT males at different ratios to wild type. (A) Test suppression cages were established with 50 wild-type males, 50 wild-type virgin females, and either 0, 50,100, 250, or 500 pgSITD15 males (for the 0:1, 1:1, 2:1, 5:1, and 10:1 pgSIT:wild-type male ratios respectively). After mating and blood feeding, the hatching rate was calculated for each cage. (B) The egg counts from population suppression assay cages. Groups 0:1 and 10:1 are significantly different (P < 0.05, Dunnett’s multiple comparisons). Mean and SD shown. (C) Population suppression as measured by the hatching rate (%) from cages suppressed by different ratios of pgSIT males to wild type males. The hatching rate is reported as the percent of eggs that hatched (n% = n 1-d-old larvae/n eggs laid). The 0:1 control group differs significantly with both the 5:1 (P < 0.001) and 10:1 (P < 0.001) groups (one-way ANOVA, Dunnett’s multiple comparisons test). Mean and SD shown. Created with Biorender.com.
Effectiveness of precision-guided sterile insect technique
The final precision-guided SIT system developed in the study produced more than 99.5% male sterility and more than 99.9% female lethality in the hybrid progeny.
The genetically sterilized male mosquitoes lacked testes but otherwise maintained a normal lower reproductive tract. These mosquitoes also exhibited good longevity. The presence of a normal lower reproductive tract revealed that these mosquitoes are able to transfer a mating plug, an essential requirement for the induction of refractoriness in females.
The scientists conducted competition cage trial assays wherein these male sterilized mosquitoes competed against wild-type male mosquitoes. The findings showed that sterilized mosquitoes are able to induce sustained population suppression.
Furthermore, the scientists modeled hypothetical releases of precision-guided SIT Anopheles gambiae eggs to explore their potential to eliminate a local Anopheles gambiae population. The findings showed that the precision-guided SIT system can eliminate local Anopheles gambiae populations and subsequently prevent malaria transmission.
Study significance
The study describes the development of a genetic SIT technology termed precision-guided SIT system that exhibits complete female elimination and near complete male sterilization. Genetically sterilized male mosquitoes produced using this system are able to induce robust population suppression and are predicted to eliminate wild Anopheles gambiae populations.
As mentioned by the scientists, this system may enable suppression of the adjacent species within the Anopheles gambiae complex, including Anopheles arabiensis, Anopheles quadriannulatus, Anopheles melas, and Anopheles merus.
This system does not aim to release transgenes into the population. However, rare fertile escapee males observed in the study suggest that some CRISPR transgenes can be released in the population. These transgenes are expected to disappear from the population because of their inherent fitness defects.
Overall, this precision-guided SIT system is a valuable addition to the malaria genetic biocontrol toolkit. It fulfills most of the criteria needed for use in SIT releases. This system can produce highly sterile male mosquitoes in mass, which can cause robust population suppression.
This system is more confinable and scalable than other genetic modification technologies developed to date for this particular malaria mosquito species. This system can significantly contribute to the eradication of deadly malaria vectors.