In a recent study published in the journal Nature Neuroscience, researchers investigated the contributions of oligodendrocytes (OLs) and neurons to amyloid-β (Aβ) plaque burden in Alzheimer's disease (AD) model mice. They found that OLs and neurons add to Aβ plaque burden, wherein excitatory projection neurons need to provide a threshold level of Aβ for rapid plaque seeding. The findings are valuable for understanding AD prevention and potentially inform therapeutic strategies.
 Study: Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Image Credit: Jose Luis Calvo / Shutterstock
Study: Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Image Credit: Jose Luis Calvo / Shutterstock
Background
AD is a progressive neurodegenerative disorder characterized by memory loss, cognitive decline, and behavioral changes. It commonly leads to dementia among older adults. It involves the accumulation of Aβ plaques and neurofibrillary tangles in the brain, leading to the death of brain cells and the deterioration of brain function. Evidence suggests that Aβ production is mainly linked to excitatory neurons (ExNs). However, recent studies suggest that other cell types may also produce Aβ. Studies have shown that cultured OLs can generate detectable levels of Aβ in vitro. Given that OL lineage cells are abundant in both gray and white matter, and myelin changes are known to be associated with AD, researchers in the present study investigated whether OLs contribute to Aβ plaque burden in vivo. They aimed to understand the role of OLs in AD, potentially revealing new insights into how different cell types contribute to the disease's progression potentially opening new avenues for treatment strategies.
About the study
The researchers analyzed single-nucleus ribonucleic acid sequencing (snRNA-seq) and single-cell RNA sequencing (scRNA-seq) datasets from wild-type mouse and human nervous systems, to analyze the expression of amyloidogenic pathway genes including APP (amyloid precursor protein), BACE1 (beta-site APP cleaving enzyme 1), PSEN1 (presenilin 1), and PSEN2 (presenilin 2) across major central nervous system (CNS) cell populations. CNS cell populations, including excitatory neurons, inhibitory neurons, oligodendrocyte precursor cells, mature oligodendrocytes, astrocytes, microglia, endothelial cells, and pericytes, were used for analysis. Further, the researchers validated the expression of APP in murine and human OLs using various techniques, including immunolabeling and in situ hybridization (ISH). Novel AD mouse lines were created to assess the contribution of OLs and ExNs to Aβ production. The APPNLGF knock-in mice were crossed with Bace1fl/fl mice to conditionally knock out (cKO) Bace1 in specific cell types using Cnp-Cre for OLs and Nex-Cre for ExNs. APP processing alterations were analyzed using Western blotting. Light sheet microscopy (LSM) was employed for imaging amyloid plaques in mouse brains, and an electrochemiluminescence assay was used to measure Aβ levels.
Results and discussion
According to the study, both neurons and OLs were found to express amyloidogenic pathway genes. APP expression in OLs was confirmed in both murine and human tissues. Novel AD mouse lines revealed a 30% reduction in plaque burden in OL-Bace1cKO;AD mice compared to controls, while ExN-Bace1cKO;AD mice showed a 95–98% reduction. This indicated that ExN-derived Aβ is crucial for plaque formation. Despite the high expression of amyloidogenic genes in OLs, their contribution to overall Aβ deposition was smaller compared to neurons.
Plaque burden was found to be nonlinearly related to Aβ production, with neuronal Aβ being essential for reaching the threshold level for plaque seeding. Residual Aβ production in ExN-Bace1cKO;AD brains was mainly from OLs. Functional analysis showed no changes in neuronal or axonal abundance due to Bace1 cKO, and myelin profiles remained unchanged. The findings suggest that while OLs contribute to Aβ production, their efficiency in generating Aβ is lower than that of neurons.
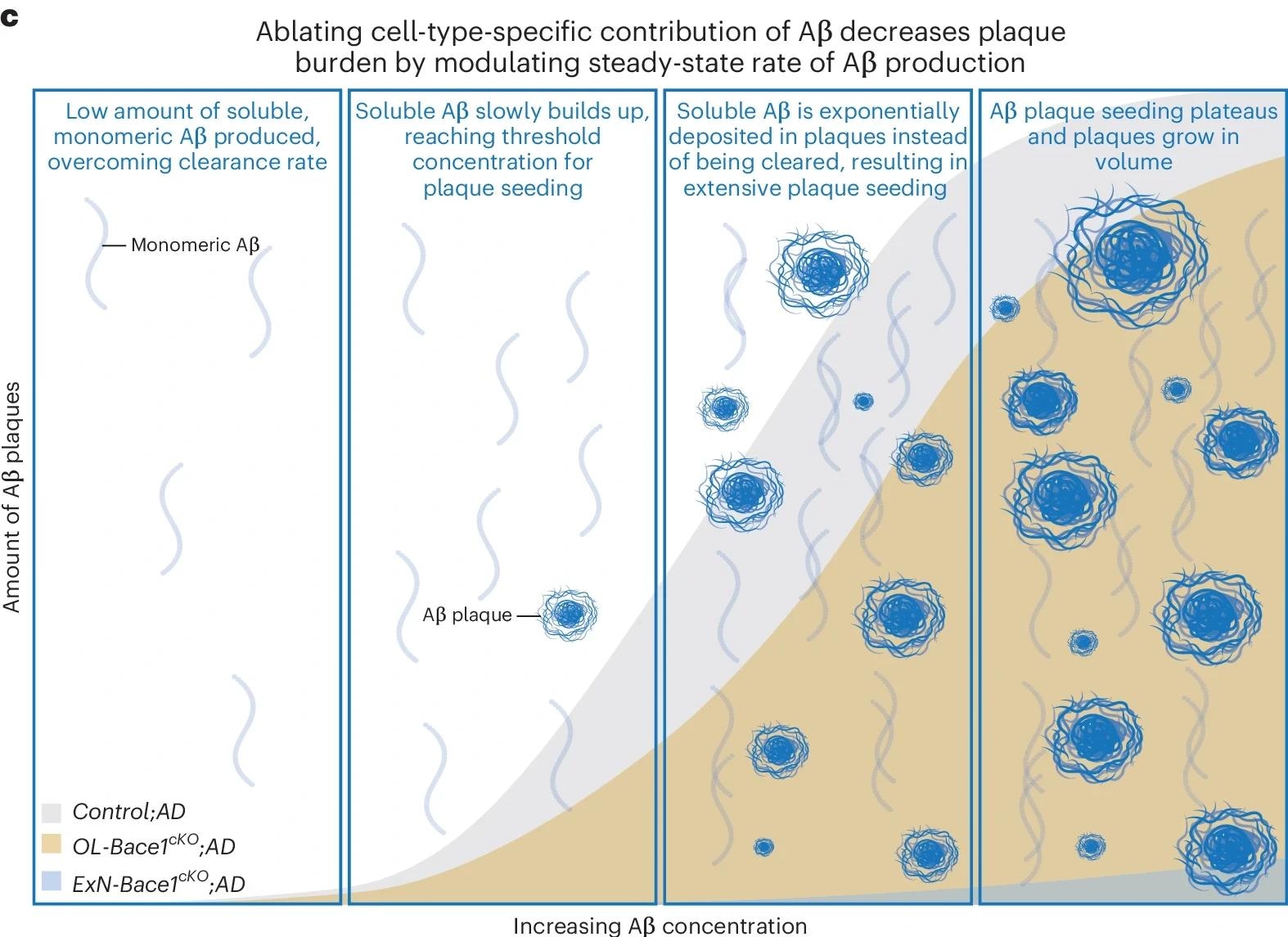 Working model of modulating cell-type-specific Aβ contributions. Selectively ablating Aβ from specific cell types results in steady-state rate change of Aβ production, causing exponentially slower plaque growth that follows a sigmoidal growth curve. ctrl, control; rel., relative.
Working model of modulating cell-type-specific Aβ contributions. Selectively ablating Aβ from specific cell types results in steady-state rate change of Aβ production, causing exponentially slower plaque growth that follows a sigmoidal growth curve. ctrl, control; rel., relative.
Conclusion
In conclusion, the study provides the first in vivo evidence that OLs significantly contribute to AD by establishing primary Aβ pathology. The reduction in plaques in mice was comparable to the effects of aducanumab and lecanemab antibody therapies. Although ExN-derived Aβ remains necessary for rapid plaque formation, the present discovery shifts some focus from solely ExNs to include OLs in AD pathology. The findings also suggest that targeting BACE1 specifically in OLs could provide a therapeutic strategy with fewer side effects compared to widespread BACE1 inhibition. Additionally, it supports the potential of BACE1 inhibitors in preventing amyloidosis if administered before Aβ reaches harmful levels, offering new insights for the development of more effective AD treatments.
Journal reference:
- Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer's disease model mice. Sasmita, A.O. et al., Nature Neuroscience (2024), DOI: 10.1038/s41593-024-01730-3, https://www.nature.com/articles/s41593-024-01730-3